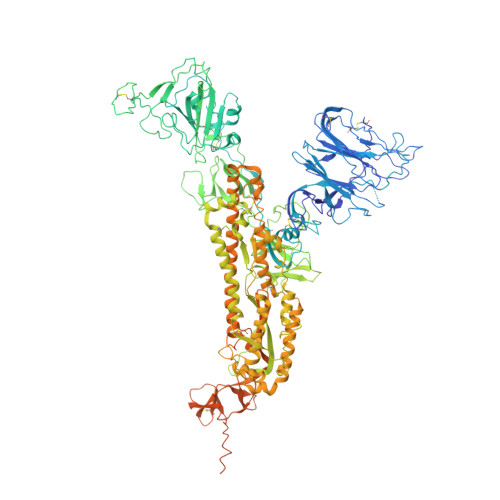
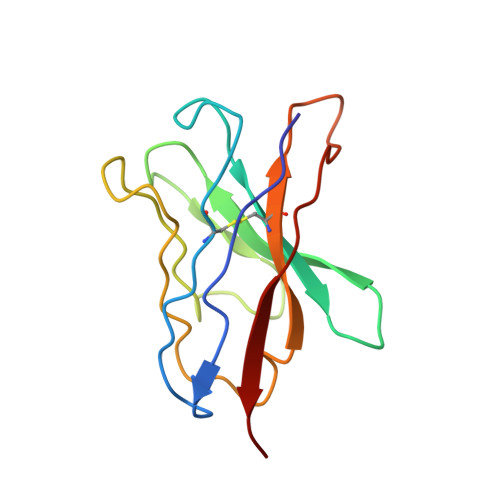
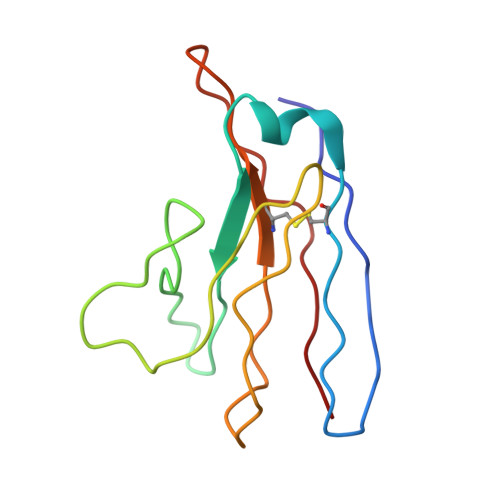
Antibody screening at reduced pH enables preferential selection of potently neutralizing antibodies targeting SARS-CoV-2.
Madan, B., Reddem, E.R., Wang, P., Casner, R.G., Nair, M.S., Huang, Y., Fahad, A.S., de Souza, M.O., Banach, B.B., Lopez Acevedo, S.N., Pan, X., Nimrania, R., Teng, I.T., Bahna, F., Zhou, T., Zhang, B., Yin, M.T., Ho, D.D., Kwong, P.D., Shapiro, L., DeKosky, B.J.(2021) AIChE J 67: e17440-e17440
- PubMed: 34898670
- DOI: https://doi.org/10.1002/aic.17440
- Primary Citation of Related Structures:
7MXP - PubMed Abstract:
Antiviral monoclonal antibody (mAb) discovery enables the development of antibody-based antiviral therapeutics. Traditional antiviral mAb discovery relies on affinity between antibody and a viral antigen to discover potent neutralizing antibodies, but these approaches are inefficient because many high affinity mAbs have no neutralizing activity. We sought to determine whether screening for anti-SARS-CoV-2 mAbs at reduced pH could provide more efficient neutralizing antibody discovery. We mined the antibody response of a convalescent COVID-19 patient at both physiological pH (7.4) and reduced pH (4.5), revealing that SARS-CoV-2 neutralizing antibodies were preferentially enriched in pH 4.5 yeast display sorts. Structural analysis revealed that a potent new antibody called LP5 targets the SARS-CoV-2 N-terminal domain supersite via a unique binding recognition mode. Our data combine with evidence from prior studies to support antibody screening at pH 4.5 to accelerate antiviral neutralizing antibody discovery.
Organizational Affiliation:
Department of Pharmaceutical Chemistry The University of Kansas Lawrence Kansas USA.