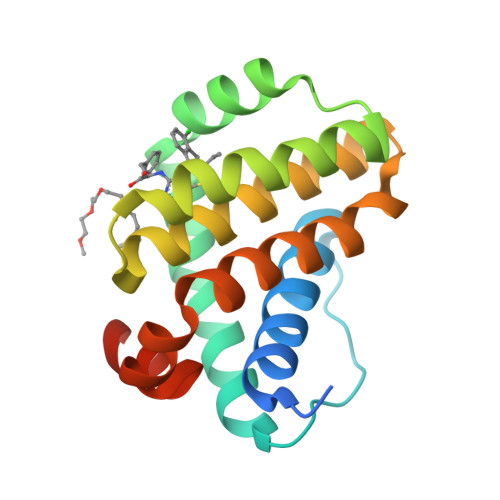
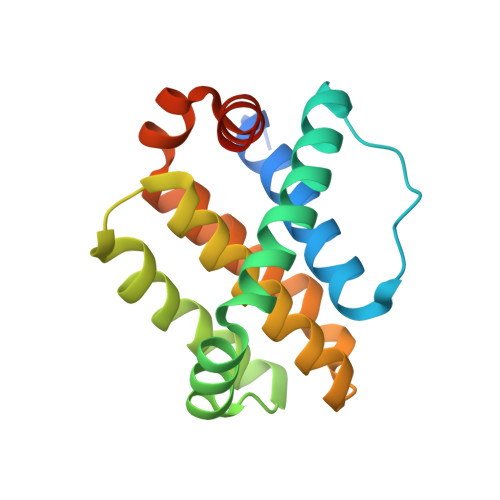
The Effect of Core Replacement on S64315, a Selective MCL-1 Inhibitor, and Its Analogues.
Sipos, S., Balint, B., Szabo, Z.B., Ondi, L., Csekei, M., Szlavik, Z., Proszenyak, A., Murray, J.B., Davidson, J., Chen, I., Dokurno, P., Surgenor, A.E., Pedder, C., Hubbard, R.E., Maragno, A.L., Chanrion, M., Colland, F., Geneste, O., Kotschy, A.(2021) ACS Omega 6: 22073-22102
- PubMed: 34497901
- DOI: https://doi.org/10.1021/acsomega.1c02595
- Primary Citation of Related Structures:
7NB4, 7NB7 - PubMed Abstract:
Following the identification of thieno[2,3- d ]pyrimidine-based selective and potent inhibitors of MCL-1, we explored the effect of core swapping at different levels of advancement. During hit-to-lead optimization, X-ray-guided S-N replacement in the core provided a new vector, whose exploration led to the opening of the so-called deep-S2 pocket of MCL-1. Unfortunately, the occupation of this region led to a plateau in affinity and had to be abandoned. As the project approached selection of a clinical candidate, a series of core swap analogues were also prepared. The affinity and cellular activity of these compounds showed a significant dependence on the core structure. In certain cases, we also observed an increased and accelerated epimerization of the atropoisomers. The most potent core replacement analogues showed considerable in vivo PD response. One compound was progressed into efficacy studies and inhibited tumor growth.
Organizational Affiliation:
Servier Research Institute of Medicinal Chemistry, Záhony u. 7., H-1031 Budapest, Hungary.