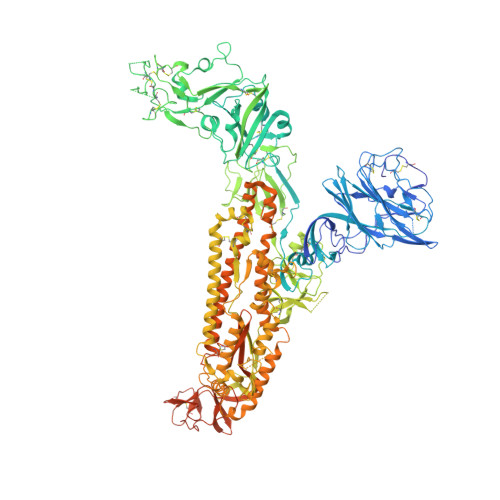
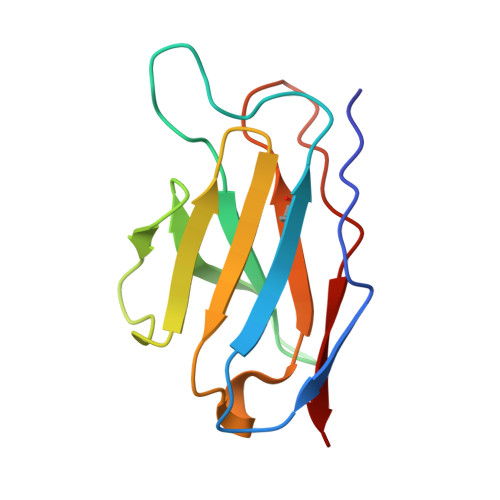
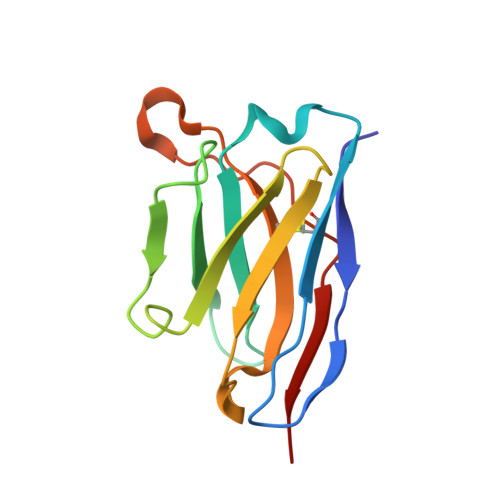
Antigenic structure of the human coronavirus OC43 spike reveals exposed and occluded neutralizing epitopes.
Wang, C., Hesketh, E.L., Shamorkina, T.M., Li, W., Franken, P.J., Drabek, D., van Haperen, R., Townend, S., van Kuppeveld, F.J.M., Grosveld, F., Ranson, N.A., Snijder, J., de Groot, R.J., Hurdiss, D.L., Bosch, B.J.(2022) Nat Commun 13: 2921-2921
- PubMed: 35614127
- DOI: https://doi.org/10.1038/s41467-022-30658-0
- Primary Citation of Related Structures:
7PNM, 7PNQ, 7PO5 - PubMed Abstract:
Human coronavirus OC43 is a globally circulating common cold virus sustained by recurrent reinfections. How it persists in the population and defies existing herd immunity is unknown. Here we focus on viral glycoprotein S, the target for neutralizing antibodies, and provide an in-depth analysis of its antigenic structure. Neutralizing antibodies are directed to the sialoglycan-receptor binding site in S1 A domain, but, remarkably, also to S1 B . The latter block infection yet do not prevent sialoglycan binding. While two distinct neutralizing S1 B epitopes are readily accessible in the prefusion S trimer, other sites are occluded such that their accessibility must be subject to conformational changes in S during cell-entry. While non-neutralizing antibodies were broadly reactive against a collection of natural OC43 variants, neutralizing antibodies generally displayed restricted binding breadth. Our data provide a structure-based understanding of protective immunity and adaptive evolution for this endemic coronavirus which emerged in humans long before SARS-CoV-2.
Organizational Affiliation:
Virology Section, Infectious Diseases and Immunology Division, Department of Biomolecular Health Sciences, Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands.