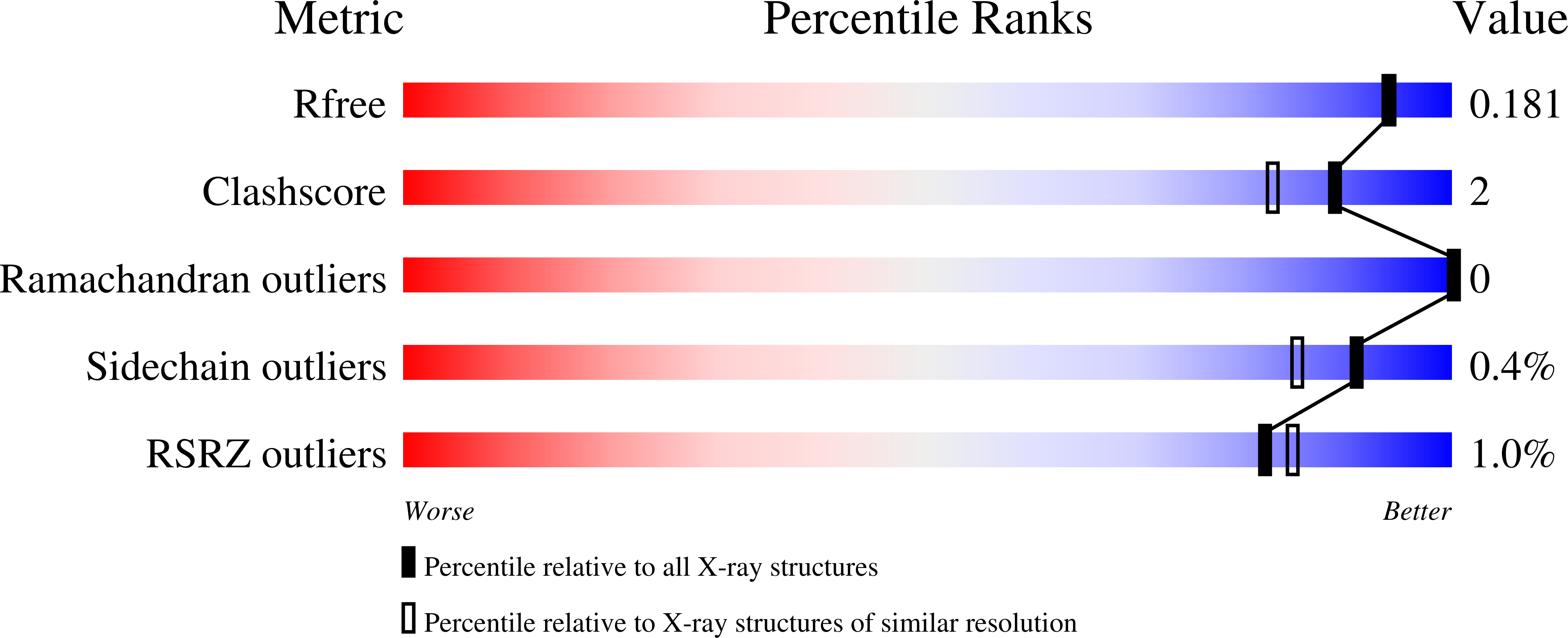
Probing the Requirements for Dual Angiotensin-Converting Enzyme C-Domain Selective/Neprilysin Inhibition.
Arendse, L.B., Cozier, G.E., Eyermann, C.J., Basarab, G.S., Schwager, S.L., Chibale, K., Acharya, K.R., Sturrock, E.D.(2022) J Med Chem 65: 3371-3387
- PubMed: 35113565
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01924
- Primary Citation of Related Structures:
7Q24, 7Q25, 7Q26, 7Q27, 7Q28, 7Q29 - PubMed Abstract:
Selective inhibition of the angiotensin-converting enzyme C-domain (cACE) and neprilysin (NEP), leaving the ACE N-domain (nACE) free to degrade bradykinin and other peptides, has the potential to provide the potent antihypertensive and cardioprotective benefits observed for nonselective dual ACE/NEP inhibitors, such as omapatrilat, without the increased risk of adverse effects. We have synthesized three 1-carboxy-3-phenylpropyl dipeptide inhibitors with nanomolar potency based on the previously reported C-domain selective ACE inhibitor lisinopril-tryptophan (LisW) to probe the structural requirements for potent dual cACE/NEP inhibition. Here we report the synthesis, enzyme kinetic data, and high-resolution crystal structures of these inhibitors bound to nACE and cACE, providing valuable insight into the factors driving potency and selectivity. Overall, these results highlight the importance of the interplay between the S 1 ' and S 2 ' subsites for ACE domain selectivity, providing guidance for future chemistry efforts toward the development of dual cACE/NEP inhibitors.
Organizational Affiliation:
Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Observatory, Cape Town 7925, South Africa.