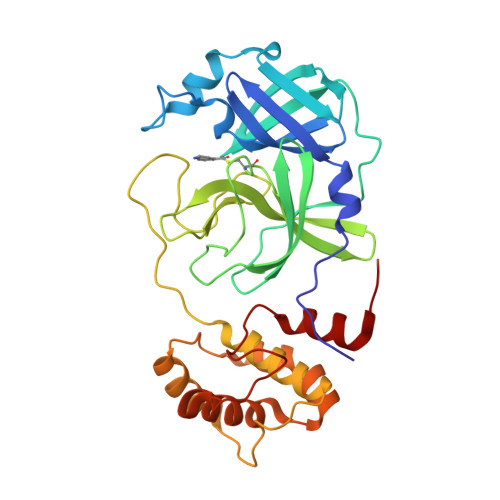
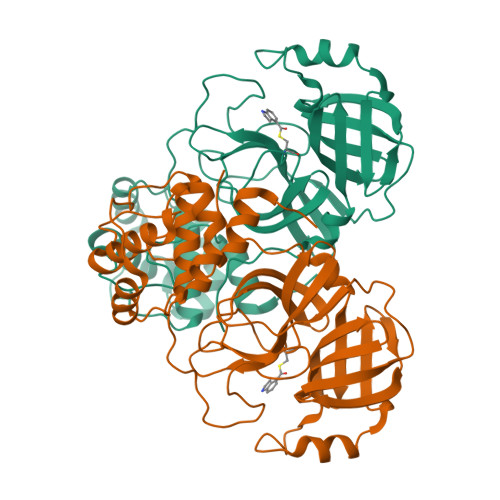
Indole Chloropyridinyl Ester-Derived SARS-CoV-2 3CLpro Inhibitors: Enzyme Inhibition, Antiviral Efficacy, Structure-Activity Relationship, and X-ray Structural Studies.
Ghosh, A.K., Raghavaiah, J., Shahabi, D., Yadav, M., Anson, B.J., Lendy, E.K., Hattori, S.I., Higashi-Kuwata, N., Mitsuya, H., Mesecar, A.D.(2021) J Med Chem 64: 14702-14714
- PubMed: 34528437
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01214
- Primary Citation of Related Structures:
7RBZ, 7RC0, 7RC1 - PubMed Abstract:
Here, we report the synthesis, structure-activity relationship studies, enzyme inhibition, antiviral activity, and X-ray crystallographic studies of 5-chloropyridinyl indole carboxylate derivatives as a potent class of SARS-CoV-2 chymotrypsin-like protease inhibitors. Compound 1 exhibited a SARS-CoV-2 3CLpro inhibitory IC 50 value of 250 nM and an antiviral EC 50 value of 2.8 μM in VeroE6 cells. Remdesivir, an RNA-dependent RNA polymerase inhibitor, showed an antiviral EC 50 value of 1.2 μM in the same assay. Compound 1 showed comparable antiviral activity with remdesivir in immunocytochemistry assays. Compound 7d with an N -allyl derivative showed the most potent enzyme inhibitory IC 50 value of 73 nM. To obtain molecular insight into the binding properties of these molecules, X-ray crystal structures of compounds 2 , 7b , and 9d -bound to SARS-CoV 3CLpro were determined, and their binding properties were compared.
Organizational Affiliation:
Department of Chemistry and Department of Medicinal Chemistry, Purdue University, West Lafayette, Indiana 47907, United States.