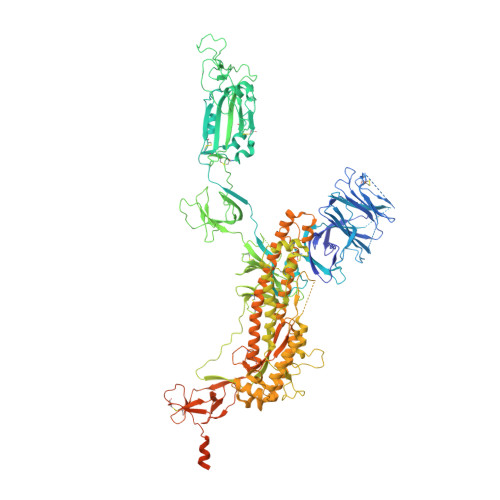
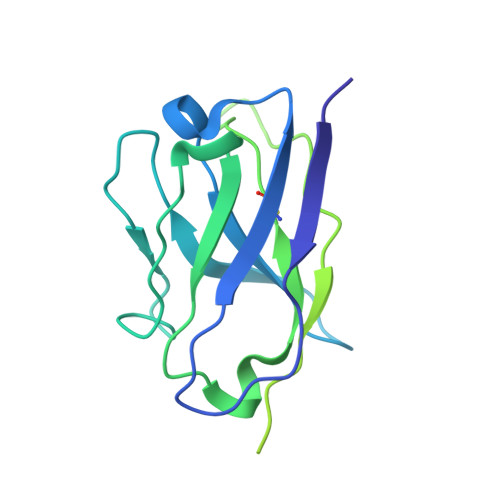
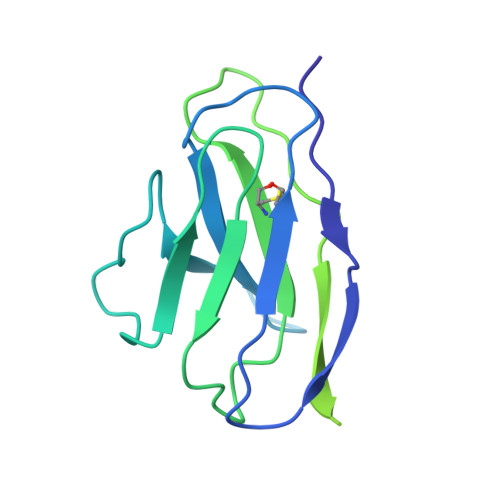
Discovery of ultrapotent broadly neutralizing antibodies from SARS-CoV-2 elite neutralizers.
Vanshylla, K., Fan, C., Wunsch, M., Poopalasingam, N., Meijers, M., Kreer, C., Kleipass, F., Ruchnewitz, D., Ercanoglu, M.S., Gruell, H., Munn, F., Pohl, K., Janicki, H., Nolden, T., Bartl, S., Stein, S.C., Augustin, M., Dewald, F., Gieselmann, L., Schommers, P., Schulz, T.F., Sander, L.E., Koch, M., Luksza, M., Lassig, M., Bjorkman, P.J., Klein, F.(2022) Cell Host Microbe 30: 69-82.e10
- PubMed: 34973165
- DOI: https://doi.org/10.1016/j.chom.2021.12.010
- Primary Citation of Related Structures:
7SC1 - PubMed Abstract:
A fraction of COVID-19 convalescent individuals mount a potent antibody response to SARS-CoV-2 with cross-reactivity to SARS-CoV-1. To uncover their humoral response in detail, we performed single B cell analysis from 10 SARS-CoV-2 elite neutralizers. We isolated and analyzed 126 monoclonal antibodies, many of which were sarbecovirus cross-reactive, with some displaying merbecovirus- and embecovirus-reactivity. Several isolated broadly neutralizing antibodies were effective against B.1.1.7, B.1.351, B.1.429, B.1.617, and B.1.617.2 variants and 19 prominent potential escape sites. Furthermore, assembly of 716,806 SARS-CoV-2 sequences predicted emerging escape variants, which were also effectively neutralized. One of these broadly neutralizing potent antibodies, R40-1G8, is a IGHV3-53 RBD-class-1 antibody. Remarkably, cryo-EM analysis revealed that R40-1G8 has a flexible binding mode, targeting both "up" and "down" conformations of the RBD. Given the threat of emerging SARS-CoV-2 variants, we demonstrate that elite neutralizers are a valuable source for isolating ultrapotent antibody candidates to prevent and treat SARS-CoV-2 infection.
Organizational Affiliation:
Institute of Virology, Faculty of Medicine and University Hospital Cologne, University of Cologne, 50931 Cologne, Germany.