Evolutionary and Structural Insights about Potential SARS-CoV-2 Evasion of Nirmatrelvir.
Yang, K.S., Leeuwon, S.Z., Xu, S., Liu, W.R.(2022) J Med Chem 65: 8686-8698
- PubMed: 35731933
- DOI: https://doi.org/10.1021/acs.jmedchem.2c00404
- Primary Citation of Related Structures:
7TE0 - PubMed Abstract:
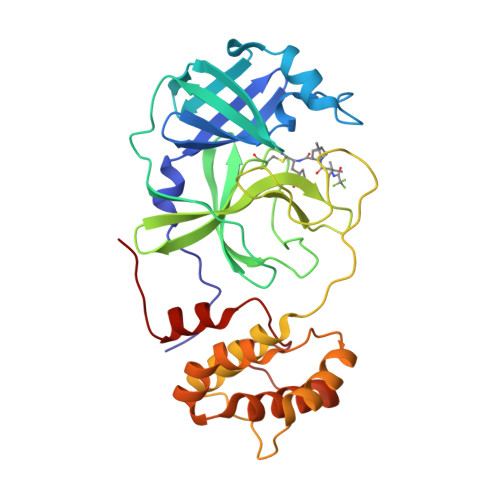
The U.S. FDA approval of PAXLOVID, a combination therapy of nirmatrelvir and ritonavir has significantly boosted our morale in fighting the COVID-19 pandemic. Nirmatrelvir is an inhibitor of the main protease (M Pro ) of SARS-CoV-2. Since many SARS-CoV-2 variants that resist vaccines and antibodies have emerged, a concern of acquired viral resistance to nirmatrelvir naturally arises. Here, possible mutations in M Pro to confer viral evasion of nirmatrelvir are analyzed and discussed from both evolutionary and structural standpoints. The analysis indicates that those mutations will likely reside in the whole aa45-51 helical region and residues including M165, L167, P168, R188, and Q189. Relevant mutations have also been observed in existing SARS-CoV-2 samples. Implications of this analysis to the fight against future drug-resistant viral variants and the development of broad-spectrum antivirals are discussed as well.
Organizational Affiliation:
Texas A&M Drug Discovery Laboratory, Department of Chemistry, Texas A&M University, College Station, Texas 77843, United States.