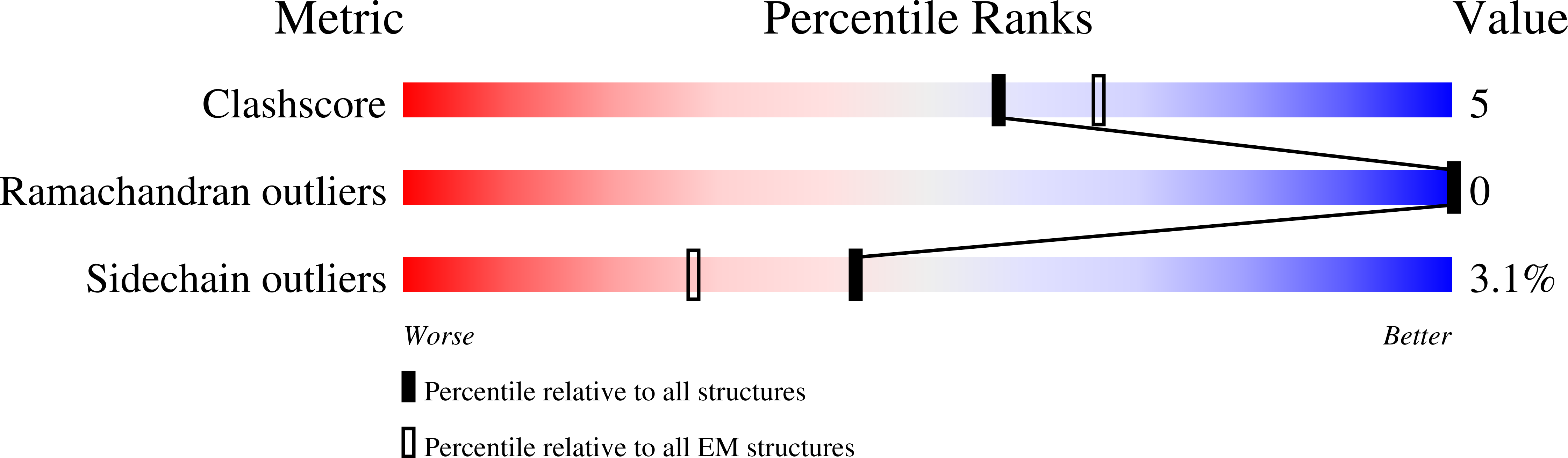Structural insights for neutralization of Omicron variants BA.1, BA.2, BA.4, and BA.5 by a broadly neutralizing SARS-CoV-2 antibody.
Kumar, S., Patel, A., Lai, L., Chakravarthy, C., Valanparambil, R., Reddy, E.S., Gottimukkala, K., Davis-Gardner, M.E., Edara, V.V., Linderman, S., Nayak, K., Dixit, K., Sharma, P., Bajpai, P., Singh, V., Frank, F., Cheedarla, N., Verkerke, H.P., Neish, A.S., Roback, J.D., Mantus, G., Goel, P.K., Rahi, M., Davis, C.W., Wrammert, J., Godbole, S., Henry, A.R., Douek, D.C., Suthar, M.S., Ahmed, R., Ortlund, E., Sharma, A., Murali-Krishna, K., Chandele, A.(2022) Sci Adv 8: eadd2032-eadd2032
- PubMed: 36197988
- DOI: https://doi.org/10.1126/sciadv.add2032
- Primary Citation of Related Structures:
7U0P, 7UPL - PubMed Abstract:
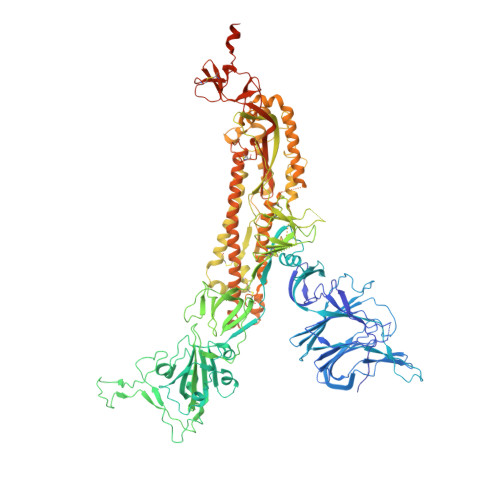
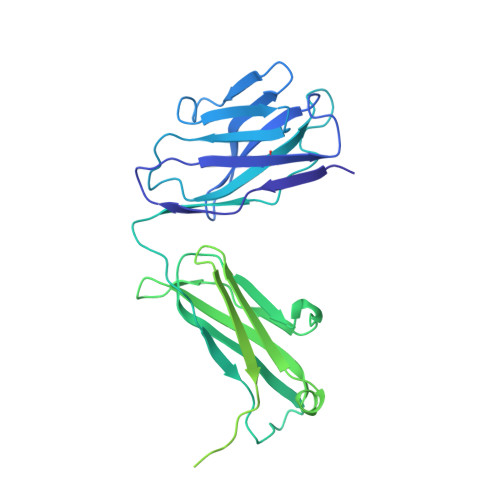
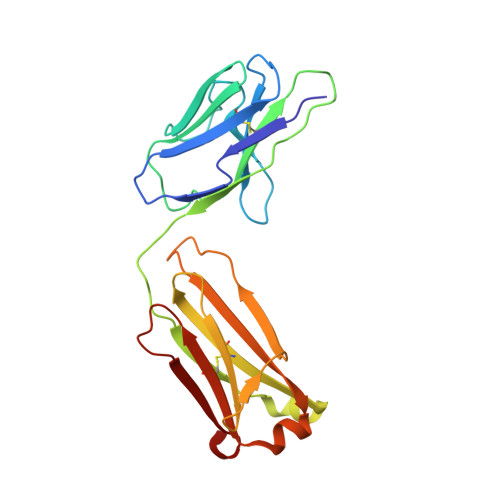
In this study, by characterizing several human monoclonal antibodies (mAbs) isolated from single B cells of the COVID-19-recovered individuals in India who experienced ancestral Wuhan strain (WA.1) of SARS-CoV-2 during early stages of the pandemic, we found a receptor binding domain (RBD)-specific mAb 002-S21F2 that has rare gene usage and potently neutralized live viral isolates of SARS-CoV-2 variants including Alpha, Beta, Gamma, Delta, and Omicron sublineages (BA.1, BA.2, BA.2.12.1, BA.4, and BA.5) with IC 50 ranging from 0.02 to 0.13 μg/ml. Structural studies of 002-S21F2 in complex with spike trimers of Omicron and WA.1 showed that it targets a conformationally conserved epitope on the outer face of RBD (class 3 surface) outside the ACE2-binding motif, thereby providing a mechanistic insights for its broad neutralization activity. The discovery of 002-S21F2 and the broadly neutralizing epitope it targets have timely implications for developing a broad range of therapeutic and vaccine interventions against SARS-CoV-2 variants including Omicron sublineages.
Organizational Affiliation:
ICGEB-Emory Vaccine Center, International Center for Genetic Engineering and Biotechnology, New Delhi-110 067, India.