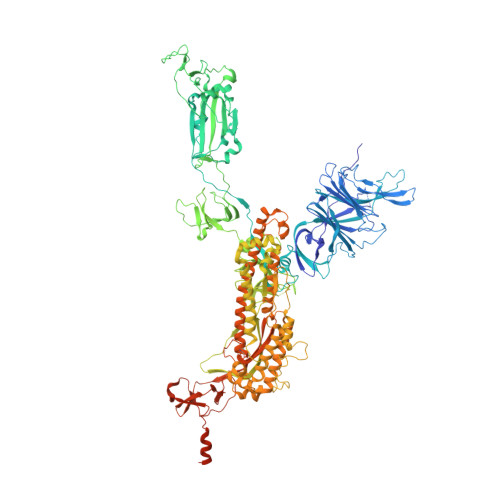
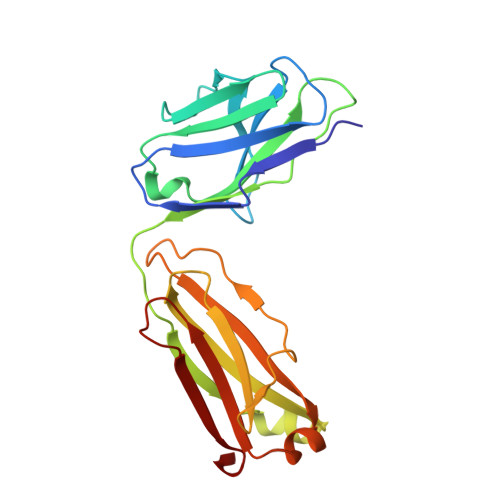
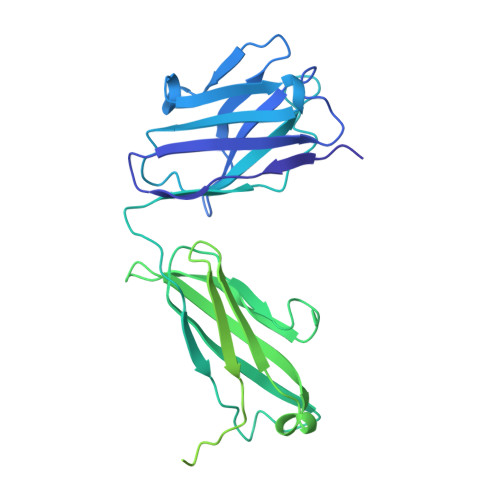
Molecular basis of SARS-CoV-2 Omicron variant evasion from shared neutralizing antibody response.
Patel, A., Kumar, S., Lai, L., Chakravarthy, C., Valanparambil, R., Reddy, E.S., Gottimukkala, K., Bajpai, P., Raju, D.R., Edara, V.V., Davis-Gardner, M.E., Linderman, S., Dixit, K., Sharma, P., Mantus, G., Cheedarla, N., Verkerke, H.P., Frank, F., Neish, A.S., Roback, J.D., Davis, C.W., Wrammert, J., Ahmed, R., Suthar, M.S., Sharma, A., Murali-Krishna, K., Chandele, A., Ortlund, E.A.(2023) Structure 31: 801-811.e5
- PubMed: 37167972
- DOI: https://doi.org/10.1016/j.str.2023.04.010
- Primary Citation of Related Structures:
7U0Q, 7U0X, 7UOW - PubMed Abstract:
Understanding the molecular features of neutralizing epitopes is important for developing vaccines/therapeutics against emerging SARS-CoV-2 variants. We describe three monoclonal antibodies (mAbs) generated from COVID-19 recovered individuals during the first wave of the pandemic in India. These mAbs had publicly shared near germline gene usage and potently neutralized Alpha and Delta, poorly neutralized Beta, and failed to neutralize Omicron BA.1 SARS-CoV-2 variants. Structural analysis of these mAbs in complex with trimeric spike protein showed that all three mAbs bivalently bind spike with two mAbs targeting class 1 and one targeting a class 4 receptor binding domain epitope. The immunogenetic makeup, structure, and function of these mAbs revealed specific molecular interactions associated with the potent multi-variant binding/neutralization efficacy. This knowledge shows how mutational combinations can affect the binding or neutralization of an antibody, which in turn relates to the efficacy of immune responses to emerging SARS-CoV-2 escape variants.
Organizational Affiliation:
Department of Biochemistry, Emory University School of Medicine, Atlanta, GA 30322, USA.