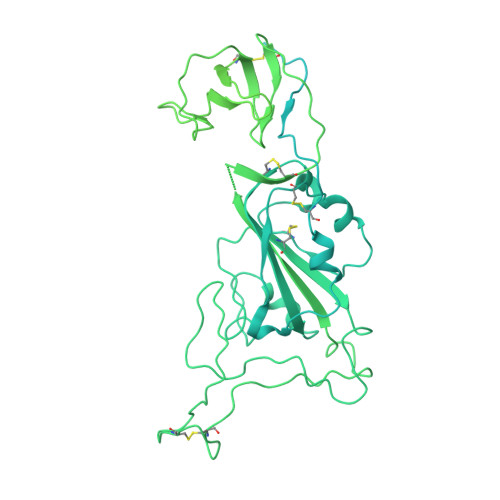
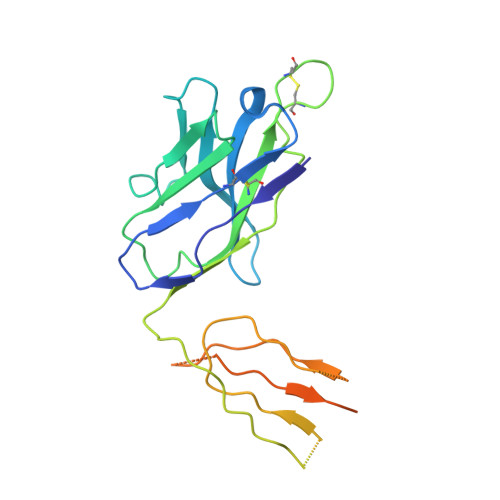
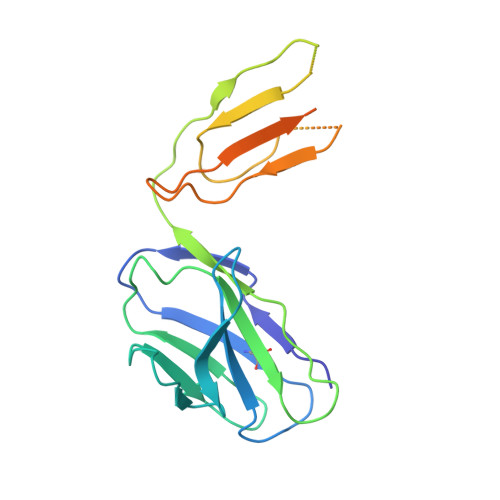
Potent monoclonal antibodies neutralize Omicron sublineages and other SARS-CoV-2 variants.
Chen, Z., Zhang, P., Matsuoka, Y., Tsybovsky, Y., West, K., Santos, C., Boyd, L.F., Nguyen, H., Pomerenke, A., Stephens, T., Olia, A.S., Zhang, B., De Giorgi, V., Holbrook, M.R., Gross, R., Postnikova, E., Garza, N.L., Johnson, R.F., Margulies, D.H., Kwong, P.D., Alter, H.J., Buchholz, U.J., Lusso, P., Farci, P.(2022) Cell Rep 41: 111528-111528
- PubMed: 36302375
- DOI: https://doi.org/10.1016/j.celrep.2022.111528
- Primary Citation of Related Structures:
7U9O, 7U9P - PubMed Abstract:
The emergence and global spread of the SARS-CoV-2 Omicron variants, which carry an unprecedented number of mutations, raise serious concerns due to the reduced efficacy of current vaccines and resistance to therapeutic antibodies. Here, we report the generation and characterization of two potent human monoclonal antibodies, NA8 and NE12, against the receptor-binding domain of the SARS-CoV-2 spike protein. NA8 interacts with a highly conserved region and has a breadth of neutralization with picomolar potency against the Beta variant and the Omicron BA.1 and BA.2 sublineages and nanomolar potency against BA.2.12.1 and BA.4. Combination of NA8 and NE12 retains potent neutralizing activity against the major SARS-CoV-2 variants of concern. Cryo-EM analysis provides the structural basis for the broad and complementary neutralizing activity of these two antibodies. We confirm the in vivo protective and therapeutic efficacies of NA8 and NE12 in the hamster model. These results show that broad and potent human antibodies can overcome the continuous immune escape of evolving SARS-CoV-2 variants.
Organizational Affiliation:
Hepatic Pathogenesis Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA. Electronic address: zchen@niaid.nih.gov.