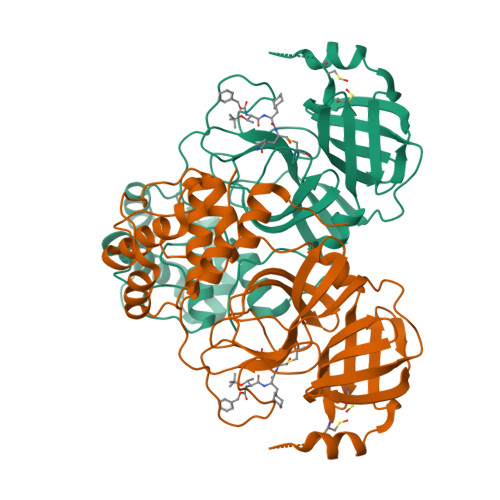
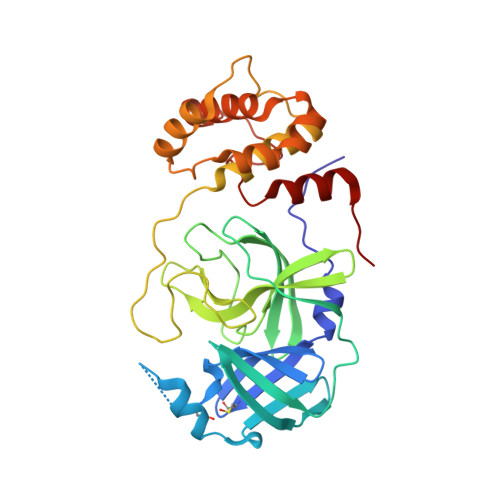
A Novel Y-Shaped, S-O-N-O-S-Bridged Cross-Link between Three Residues C22, C44, and K61 Is Frequently Observed in the SARS-CoV-2 Main Protease.
Yang, K.S., Blankenship, L.R., Kuo, S.A., Sheng, Y.J., Li, P., Fierke, C.A., Russell, D.H., Yan, X., Xu, S., Liu, W.R.(2023) ACS Chem Biol 18: 449-455
- PubMed: 36629751
- DOI: https://doi.org/10.1021/acschembio.2c00695
- Primary Citation of Related Structures:
7UU6, 7UU7, 7UU8, 7UU9, 7UUA, 7UUB, 7UUC, 7UUD, 7UUE - PubMed Abstract:
As the COVID-19 pathogen, SARS-CoV-2 relies on its main protease (M Pro ) for pathogenesis and replication. During crystallographic analyses of M Pro crystals that were exposed to the air, a uniquely Y-shaped, S-O-N-O-S-bridged post-translational cross-link that connects three residues C22, C44, and K61 at their side chains was frequently observed. As a novel covalent modification, this cross-link serves potentially as a redox switch to regulate the catalytic activity of M Pro , a demonstrated drug target of COVID-19. The formation of this linkage leads to a much more open active site that can potentially be targeted for the development of novel SARS-CoV-2 antivirals. The structural rearrangement of M Pro by this cross-link indicates that small molecules that lock M Pro in the cross-linked form can potentially be used with other active-site-targeting molecules such as paxlovid for synergistic effects in inhibiting SARS-CoV-2 viral replication.
Organizational Affiliation:
Department of Biochemistry, Brandeis University, Waltham, Massachusetts 02453, United States.