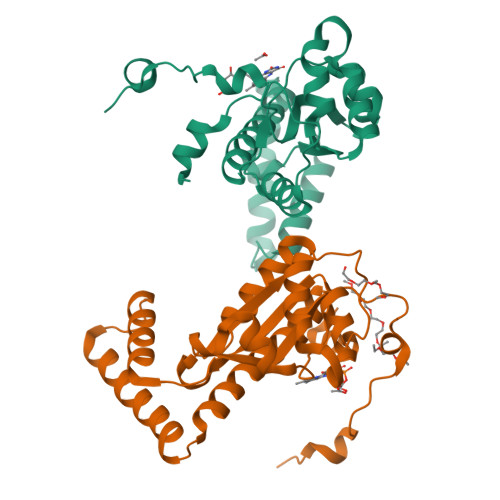
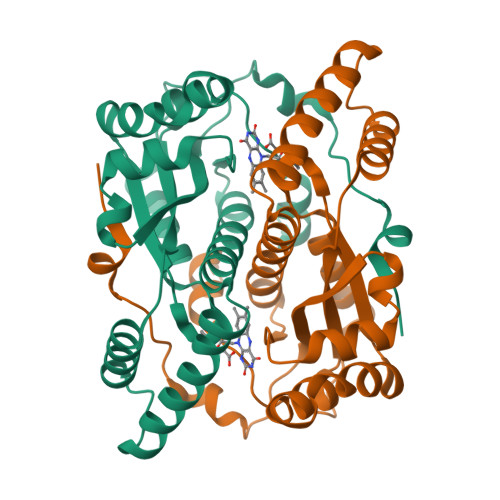
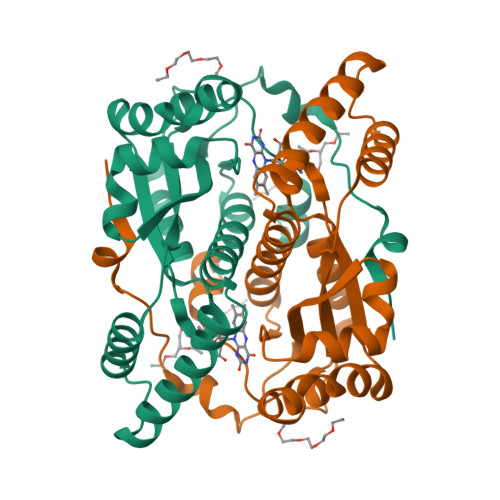
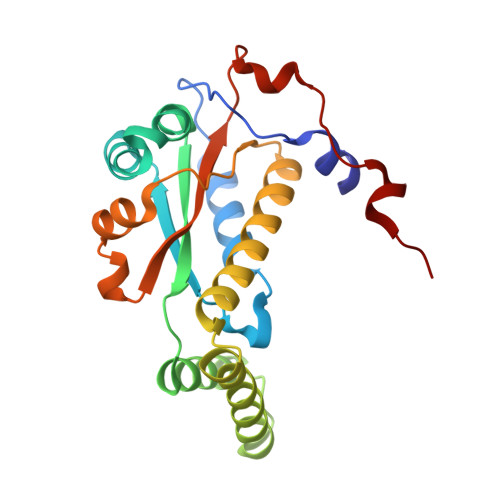
The Crystal Structure of Engineered Nitroreductase NTR 2.0 and Impact of F70A and F108Y Substitutions on Substrate Specificity.
Sharrock, A.V., Mumm, J.S., Bagdziunas, G., Cenas, N., Arcus, V.L., Ackerley, D.F.(2023) Int J Mol Sci 24
- PubMed: 37047605
- DOI: https://doi.org/10.3390/ijms24076633
- Primary Citation of Related Structures:
7UWT - PubMed Abstract:
Bacterial nitroreductase enzymes that convert prodrugs to cytotoxins are valuable tools for creating transgenic targeted ablation models to study cellular function and cell-specific regeneration paradigms. We recently engineered a nitroreductase ("NTR 2.0") for substantially enhanced reduction of the prodrug metronidazole, which permits faster cell ablation kinetics, cleaner interrogations of cell function, ablation of previously recalcitrant cell types, and extended ablation paradigms useful for modelling chronic diseases. To provide insight into the enhanced enzymatic mechanism of NTR 2.0, we have solved the X-ray crystal structure at 1.85 Angstroms resolution and compared it to the parental enzyme, NfsB from Vibrio vulnificus . We additionally present a survey of reductive activity with eight alternative nitroaromatic substrates, to provide access to alternative ablation prodrugs, and explore applications such as remediation of dinitrotoluene pollutants. The predicted binding modes of four key substrates were investigated using molecular modelling.
Organizational Affiliation:
School of Biological Sciences, Victoria University of Wellington, Wellington 6012, New Zealand.