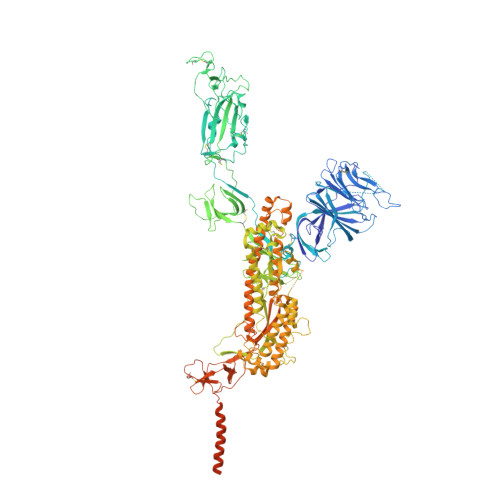
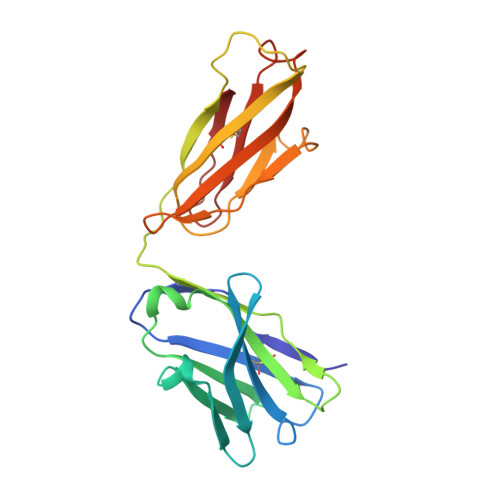
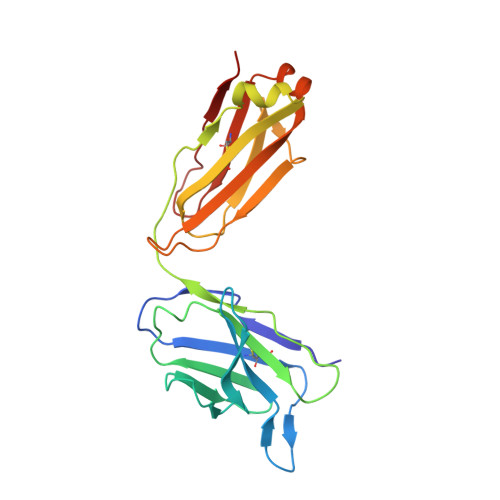
Mapping cross-variant neutralizing sites on the SARS-CoV-2 spike protein.
Xu, S., Wang, Y., Wang, Y., Zhang, C., Hong, Q., Gu, C., Xu, R., Wang, T., Yang, Y., Zang, J., Zhou, Y., Li, Z., Liu, Q., Zhou, B., Bai, L., Zhu, Y., Deng, Q., Wang, H., Lavillette, D., Wong, G., Xie, Y., Cong, Y., Huang, Z.(2022) Emerg Microbes Infect 11: 351-367
- PubMed: 34964428
- DOI: https://doi.org/10.1080/22221751.2021.2024455
- Primary Citation of Related Structures:
7WCR, 7WCZ, 7WD0, 7WD7, 7WD8, 7WD9, 7WDF - PubMed Abstract:
The emergence of multiple severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern threatens the efficacy of currently approved vaccines and authorized therapeutic monoclonal antibodies (MAbs). It is hence important to continue searching for SARS-CoV-2 broadly neutralizing MAbs and defining their epitopes. Here, we isolate 9 neutralizing mouse MAbs raised against the spike protein of a SARS-CoV-2 prototype strain and evaluate their neutralizing potency towards a panel of variants, including B.1.1.7, B.1.351, B.1.617.1, and B.1.617.2. By using a combination of biochemical, virological, and cryo-EM structural analyses, we identify three types of cross-variant neutralizing MAbs, represented by S5D2, S5G2, and S3H3, respectively, and further define their epitopes. S5D2 binds the top lateral edge of the receptor-binding motif within the receptor-binding domain (RBD) with a binding footprint centred around the loop 477-489 , and efficiently neutralizes all variant pseudoviruses, but the potency against B.1.617.2 was observed to decrease significantly. S5G2 targets the highly conserved RBD core region and exhibits comparable neutralization towards the variant panel. S3H3 binds a previously unreported epitope located within the evolutionarily stable SD1 region and is able to near equally neutralize all of the variants tested. Our work thus defines three distinct cross-variant neutralizing sites on the SARS-CoV-2 spike protein, providing guidance for design and development of broadly effective vaccines and MAb-based therapies.
Organizational Affiliation:
CAS Key Laboratory of Molecular Virology & Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences, University of Chinese Academy of Sciences, Shanghai, People's Republic of China.