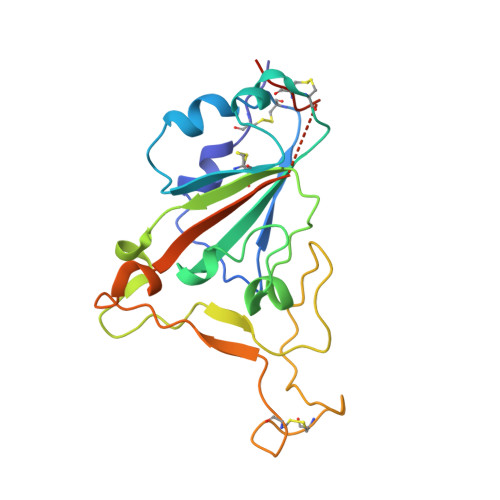
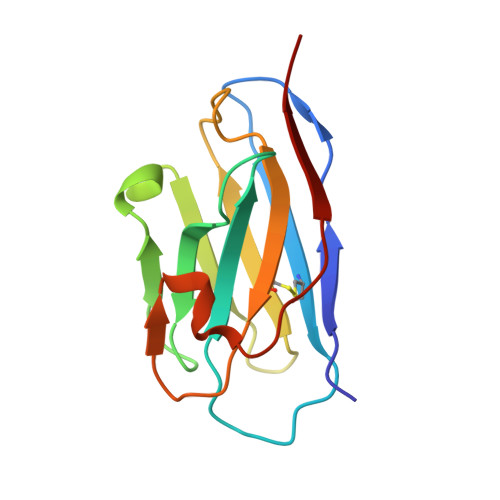
A bispecific nanobody dimer broadly neutralizes SARS-CoV-1 & 2 variants of concern and offers substantial protection against Omicron via low-dose intranasal administration.
Ma, H., Zhang, X., Zeng, W., Zhou, J., Chi, X., Chen, S., Zheng, P., Wang, M., Wu, Y., Zhao, D., Gong, F., Lin, H., Sun, H., Yu, C., Shi, Z., Hu, X., Zhang, H., Jin, T., Chiu, S.(2022) Cell Discov 8: 132-132
- PubMed: 36494344
- DOI: https://doi.org/10.1038/s41421-022-00497-w
- Primary Citation of Related Structures:
7X4I - PubMed Abstract:
Current SARS-CoV-2 Omicron subvariants impose a heavy burden on global health systems by evading immunity from most developed neutralizing antibodies and vaccines. Here, we identified a nanobody (aSA3) that strongly cross-reacts with the receptor binding domain (RBD) of both SARS-CoV-1 and wild-type (WT) SARS-CoV-2. The dimeric construct of aSA3 (aSA3-Fc) tightly binds and potently neutralizes both SARS-CoV-1 and WT SARS-CoV-2. Based on X-ray crystallography, we engineered a bispecific nanobody dimer (2-3-Fc) by fusing aSA3-Fc to aRBD-2, a previously identified broad-spectrum nanobody targeting an RBD epitope distinct from aSA3. 2-3-Fc exhibits single-digit ng/mL neutralizing potency against all major variants of concerns including BA.5. In hamsters, a single systemic dose of 2-3-Fc at 10 mg/kg conferred substantial efficacy against Omicron infection. More importantly, even at three low doses of 0.5 mg/kg, 2-3-Fc prophylactically administered through the intranasal route drastically reduced viral RNA loads and completely eliminated infectious Omicron particles in the trachea and lungs. Finally, we discovered that 2(Y29G)-3-Fc containing a Y29G substitution in aRBD-2 showed better activity than 2-3-Fc in neutralizing BA.2.75, a recent Omicron subvariant that emerged in India. This study expands the arsenal against SARS-CoV-1, provides potential therapeutic and prophylactic candidates that fully cover major SARS-CoV-2 variants, and may offer a simple preventive approach against Omicron and its subvariants.
Organizational Affiliation:
Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China.