Potent pan huACE2-dependent sarbecovirus neutralizing monoclonal antibodies isolated from a BNT162b2-vaccinated SARS survivor.
Chia, W.N., Tan, C.W., Tan, A.W.K., Young, B., Starr, T.N., Lopez, E., Fibriansah, G., Barr, J., Cheng, S., Yeoh, A.Y., Yap, W.C., Lim, B.L., Ng, T.S., Sia, W.R., Zhu, F., Chen, S., Zhang, J., Kwek, M.S.S., Greaney, A.J., Chen, M., Au, G.G., Paradkar, P.N., Peiris, M., Chung, A.W., Bloom, J.D., Lye, D., Lok, S., Wang, L.F.(2023) Sci Adv 9: eade3470-eade3470
- PubMed: 37494438
- DOI: https://doi.org/10.1126/sciadv.ade3470
- Primary Citation of Related Structures:
7Y71, 7Y72 - PubMed Abstract:
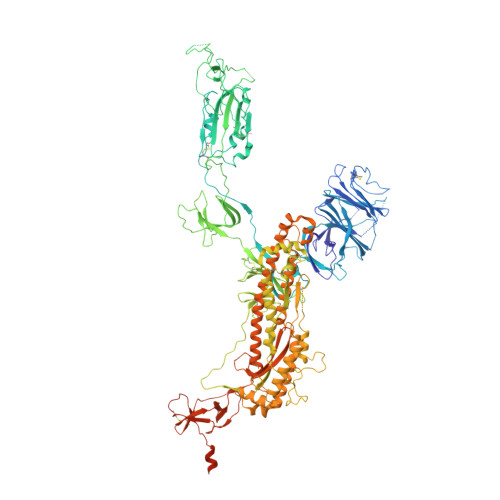
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern such as Omicron hampered efforts in controlling the ongoing coronavirus disease 2019 pandemic due to their ability to escape neutralizing antibodies induced by vaccination or prior infection, highlighting the need to develop broad-spectrum vaccines and therapeutics. Most human monoclonal antibodies (mAbs) reported to date have not demonstrated true pan-sarbecovirus neutralizing breadth especially against animal sarbecoviruses. Here, we report the isolation and characterization of highly potent mAbs targeting the receptor binding domain (RBD) of huACE2-dependent sarbecovirus from a SARS-CoV survivor vaccinated with BNT162b2. Among the six mAbs identified, one (E7) showed better huACE2-dependent sarbecovirus neutralizing potency and breadth than any other mAbs reported to date. Mutagenesis and cryo-electron microscopy studies indicate that these mAbs have a unique RBD contact footprint and that E7 binds to a quaternary structure-dependent epitope.
Organizational Affiliation:
Programme in Emerging Infectious Diseases, Duke-NUS Medical School, Singapore, Singapore.