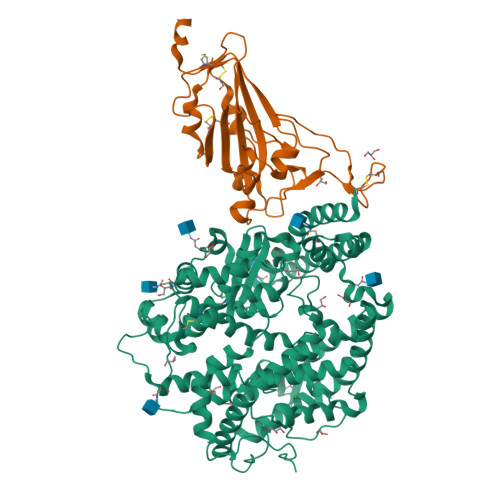
A delicate balance between antibody evasion and ACE2 affinity for Omicron BA.2.75.
Huo, J., Dijokaite-Guraliuc, A., Liu, C., Zhou, D., Ginn, H.M., Das, R., Supasa, P., Selvaraj, M., Nutalai, R., Tuekprakhon, A., Duyvesteyn, H.M.E., Mentzer, A.J., Skelly, D., Ritter, T.G., Amini, A., Bibi, S., Adele, S., Johnson, S.A., Paterson, N.G., Williams, M.A., Hall, D.R., Plowright, M., Newman, T.A.H., Hornsby, H., de Silva, T.I., Temperton, N., Klenerman, P., Barnes, E., Dunachie, S.J., Pollard, A.J., Lambe, T., Goulder, P., Fry, E.E., Mongkolsapaya, J., Ren, J., Stuart, D.I., Screaton, G.R.(2022) Cell Rep 42: 111903-111903
- PubMed: 36586406
- DOI: https://doi.org/10.1016/j.celrep.2022.111903
- Primary Citation of Related Structures:
8ASY - PubMed Abstract:
Variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have caused successive global waves of infection. These variants, with multiple mutations in the spike protein, are thought to facilitate escape from natural and vaccine-induced immunity and often increase in affinity for ACE2. The latest variant to cause concern is BA.2.75, identified in India where it is now the dominant strain, with evidence of wider dissemination. BA.2.75 is derived from BA.2 and contains four additional mutations in the receptor-binding domain (RBD). Here, we perform an antigenic and biophysical characterization of BA.2.75, revealing an interesting balance between humoral evasion and ACE2 receptor affinity. ACE2 affinity for BA.2.75 is increased 9-fold compared with BA.2; there is also evidence of escape of BA.2.75 from immune serum, particularly that induced by Delta infection, which may explain the rapid spread in India, where where there is a high background of Delta infection. ACE2 affinity appears to be prioritized over greater escape.
Organizational Affiliation:
State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China; Division of Structural Biology, Nuffield Department of Medicine, University of Oxford, the Wellcome Centre for Human Genetics, Oxford, UK; Guangzhou Laboratory, Bio-island, Guangzhou 510320, China. Electronic address: huojiandong@gird.cn.