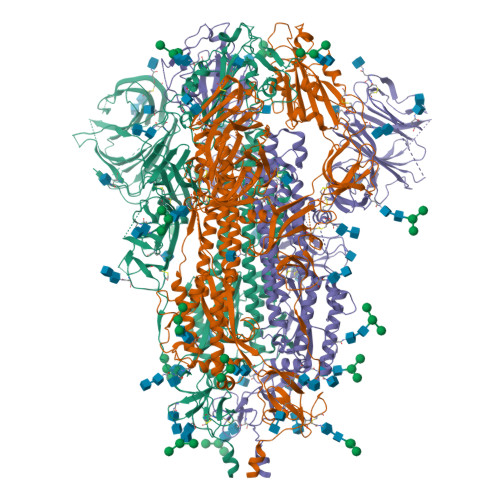
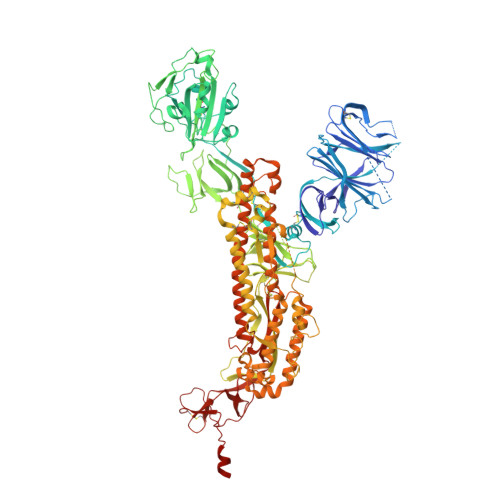
Structural and computational design of a SARS-CoV-2 spike antigen with improved expression and immunogenicity.
Williams, J.A., Biancucci, M., Lessen, L., Tian, S., Balsaraf, A., Chen, L., Chesterman, C., Maruggi, G., Vandepaer, S., Huang, Y., Mallett, C.P., Steff, A.M., Bottomley, M.J., Malito, E., Wahome, N., Harshbarger, W.D.(2023) Sci Adv 9: eadg0330-eadg0330
- PubMed: 37285422
- DOI: https://doi.org/10.1126/sciadv.adg0330
- Primary Citation of Related Structures:
8EPN, 8EPP, 8EPQ - PubMed Abstract:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern challenge the efficacy of approved vaccines, emphasizing the need for updated spike antigens. Here, we use an evolutionary-based design aimed at boosting protein expression levels of S-2P and improving immunogenic outcomes in mice. Thirty-six prototype antigens were generated in silico and 15 were produced for biochemical analysis. S2D14, which contains 20 computationally designed mutations within the S2 domain and a rationally engineered D614G mutation in the SD2 domain, has an ~11-fold increase in protein yield and retains RBD antigenicity. Cryo-electron microscopy structures reveal a mixture of populations in various RBD conformational states. Vaccination of mice with adjuvanted S2D14 elicited higher cross-neutralizing antibody titers than adjuvanted S-2P against the SARS-CoV-2 Wuhan strain and four variants of concern. S2D14 may be a useful scaffold or tool for the design of future coronavirus vaccines, and the approaches used for the design of S2D14 may be broadly applicable to streamline vaccine discovery.
Organizational Affiliation:
GSK, Rockville, MD, USA.