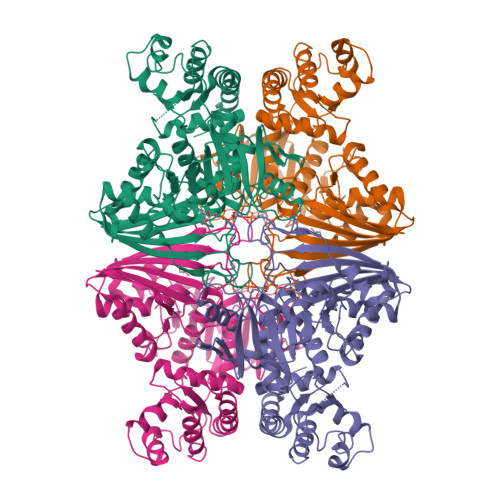
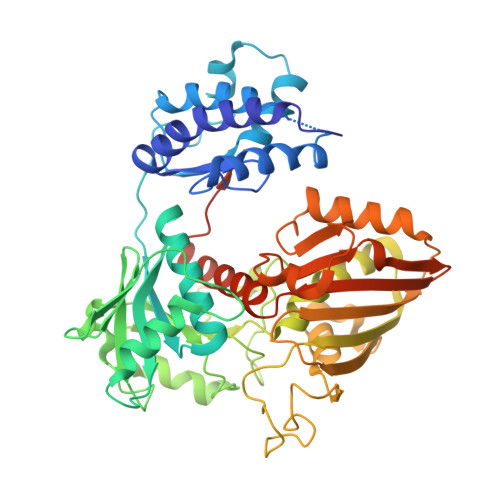
Structure-based functional analysis of a novel NADPH-producing glyceraldehyde-3-phosphate dehydrogenase from Corynebacterium glutamicum.
Son, H.F., Park, W., Kim, S., Kim, I.K., Kim, K.J.(2023) Int J Biol Macromol 255: 128103-128103
- PubMed: 37992937
- DOI: https://doi.org/10.1016/j.ijbiomac.2023.128103
- Primary Citation of Related Structures:
8JB1 - PubMed Abstract:
Corynebacterium glutamicum is an industrial workhorse applied in the production of valuable biochemicals. In the process of bio-based chemical production, improving cofactor recycling and mitigating cofactor imbalance are considered major solutions for enhancing the production yield and efficiency. Although, glyceraldehyde-3-phosphate dehydrogenase (GapDH), a glycolytic enzyme, can be a promising candidate for a sufficient NADPH cofactor supply, however, most microorganisms have only NAD-dependent GapDHs. In this study, we performed functional characterization and structure determination of novel NADPH-producing GapDH from C. glutamicum (CgGapX). Based on the crystal structure of CgGapX in complex with NADP cofactor, the unique structural features of CgGapX for NADP stabilization were elucidated. Also, N-terminal additional region (Auxiliary domain, AD) appears to have an effect on enzyme stabilization. In addition, through structure-guided enzyme engineering, we developed a CgGapX variant that exhibited 4.3-fold higher k cat , and 1.2-fold higher k cat /K M values when compared with wild-type. Furthermore, a bioinformatic analysis of 100 GapX-like enzymes from 97 microorganisms in the KEGG database revealed that the GapX-like enzymes possess a variety of AD, which seem to determine enzyme stability. Our findings are expected to provide valuable information for supplying NADPH cofactor pools in bio-based value-added chemical production.
Organizational Affiliation:
Clean Energy Research Center, Korea Institute of Science and Technology, Seoul 02792, Republic of Korea.