Heterologous expression, purification and structural features of native Dictyostelium discoideum dye-decolorizing peroxidase bound to a natively incorporated heme.
Kalkan, O., Kantamneni, S., Brings, L., Han, H., Bean, R., Mancuso, A.P., Koua, F.H.M.(2023) Front Chem 11: 1220543-1220543
- PubMed: 37593106
- DOI: https://doi.org/10.3389/fchem.2023.1220543
- Primary Citation of Related Structures:
8OHY - PubMed Abstract:
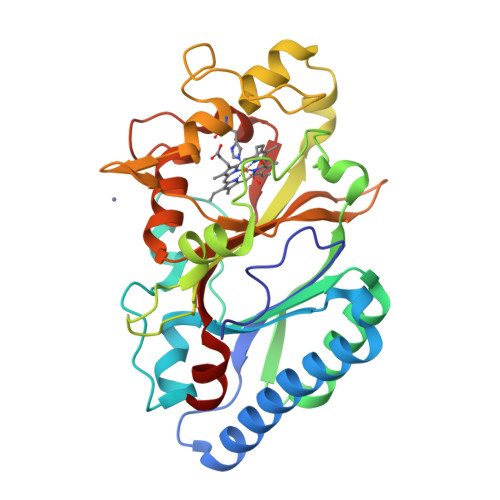
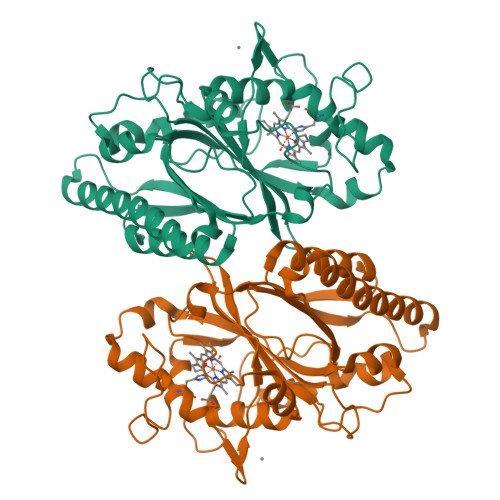
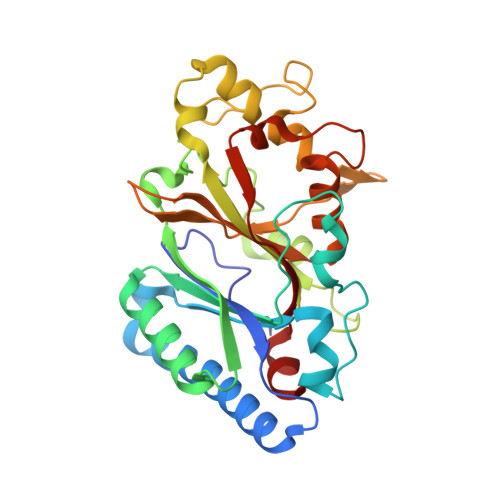
The Dictyostelium discoideum dye-decolorizing peroxidase ( Dd DyP) is a newly discovered peroxidase, which belongs to a unique class of heme peroxidase family that lacks homology to the known members of plant peroxidase superfamily. Dd DyP catalyzes the H 2 O 2 -dependent oxidation of a wide-spectrum of substrates ranging from polycyclic dyes to lignin biomass, holding promise for potential industrial and biotechnological applications. To study the molecular mechanism of Dd DyP, highly pure and functional protein with a natively incorporated heme is required, however, obtaining a functional DyP-type peroxidase with a natively bound heme is challenging and often requires addition of expensive biosynthesis precursors. Alternatively, a heme in vitro reconstitution approach followed by a chromatographic purification step to remove the excess heme is often used. Here, we show that expressing the Dd DyP peroxidase in ×2 YT enriched medium at low temperature (20°C), without adding heme supplement or biosynthetic precursors, allows for a correct native incorporation of heme into the apo-protein, giving rise to a stable protein with a strong Soret peak at 402 nm. Further, we crystallized and determined the native structure of Dd DyP at a resolution of 1.95 Å, which verifies the correct heme binding and its geometry. The structural analysis also reveals a binding of two water molecules at the distal site of heme plane bridging the catalytic residues (Arg239 and Asp149) of the GXXDG motif to the heme-Fe(III) via hydrogen bonds. Our results provide new insights into the geometry of native Dd DyP active site and its implication on DyP catalysis.
Organizational Affiliation:
European XFEL GmbH, Schenefeld, Schleswig-Holstein, Germany.