Discovery and Crystallographic Studies of Trisubstituted Piperazine Derivatives as Non-Covalent SARS-CoV-2 Main Protease Inhibitors with High Target Specificity and Low Toxicity.
Gao, S., Sylvester, K., Song, L., Claff, T., Jing, L., Woodson, M., Weisse, R.H., Cheng, Y., Schakel, L., Petry, M., Gutschow, M., Schiedel, A.C., Strater, N., Kang, D., Xu, S., Toth, K., Tavis, J., Tollefson, A.E., Muller, C.E., Liu, X., Zhan, P.(2022) J Med Chem 65: 13343-13364
- PubMed: 36107752
- DOI: https://doi.org/10.1021/acs.jmedchem.2c01146
- Primary Citation of Related Structures:
8ACD, 8ACL - PubMed Abstract:
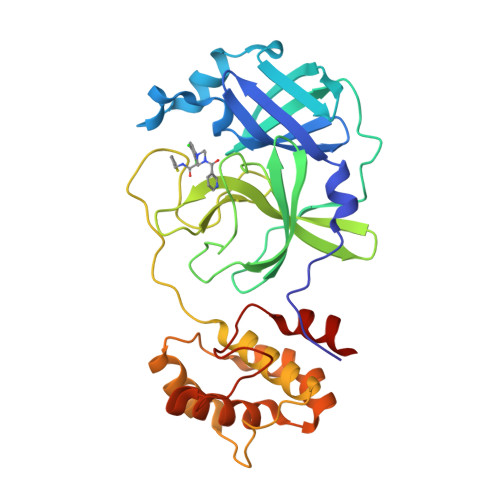
The continuous spread of SARS-CoV-2 calls for more direct-acting antiviral agents to combat the highly infectious variants. The main protease (M pro ) is an promising target for anti-SARS-CoV-2 drug design. Here, we report the discovery of potent non-covalent non-peptide M pro inhibitors featuring a 1,2,4-trisubstituted piperazine scaffold. We systematically modified the non-covalent hit MCULE-5948770040 by structure-based rational design combined with multi-site binding and privileged structure assembly strategies. The optimized compound GC-14 inhibits M pro with high potency (IC 50 = 0.40 μM) and displays excellent antiviral activity (EC 50 = 1.1 μM), being more potent than Remdesivir. Notably, GC-14 exhibits low cytotoxicity (CC 50 > 100 μM) and excellent target selectivity for SARS-CoV-2 M pro (IC 50 > 50 μM for cathepsins B, F, K, L, and caspase 3). X-ray co-crystal structures prove that the inhibitors occupy multiple subpockets by critical non-covalent interactions. These studies may provide a basis for developing a more efficient and safer therapy for COVID-19.
Organizational Affiliation:
Department of Medicinal Chemistry, Key Laboratory of Chemical Biology, Ministry of Education, School of Pharmaceutical Sciences, Shandong University, Ji'nan 250012, China.