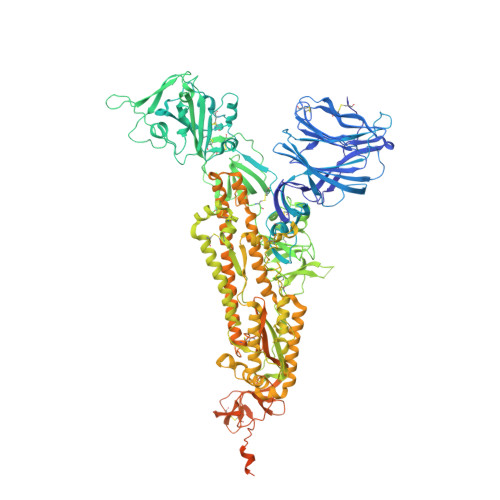
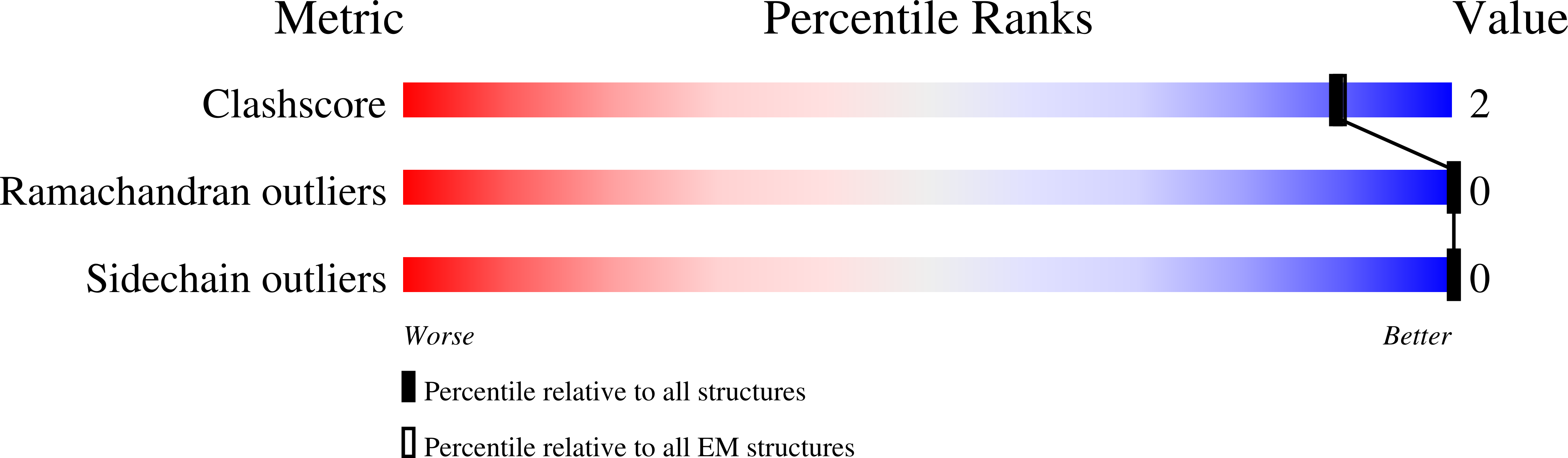Design, structure and plasma binding of ancestral beta-CoV scaffold antigens.
Hueting, D., Schriever, K., Sun, R., Vlachiotis, S., Zuo, F., Du, L., Persson, H., Hofstrom, C., Ohlin, M., Wallden, K., Buggert, M., Hammarstrom, L., Marcotte, H., Pan-Hammarstrom, Q., Andrell, J., Syren, P.O.(2023) Nat Commun 14: 6527-6527
- PubMed: 37845250
- DOI: https://doi.org/10.1038/s41467-023-42200-x
- Primary Citation of Related Structures:
8AJA, 8AJL - PubMed Abstract:
We report the application of ancestral sequence reconstruction on coronavirus spike protein, resulting in stable and highly soluble ancestral scaffold antigens (AnSAs). The AnSAs interact with plasma of patients recovered from COVID-19 but do not bind to the human angiotensin-converting enzyme 2 (ACE2) receptor. Cryo-EM analysis of the AnSAs yield high resolution structures (2.6-2.8 Å) indicating a closed pre-fusion conformation in which all three receptor-binding domains (RBDs) are facing downwards. The structures reveal an intricate hydrogen-bonding network mediated by well-resolved loops, both within and across monomers, tethering the N-terminal domain and RBD together. We show that AnSA-5 can induce and boost a broad-spectrum immune response against the wild-type RBD as well as circulating variants of concern in an immune organoid model derived from tonsils. Finally, we highlight how AnSAs are potent scaffolds by replacing the ancestral RBD with the wild-type sequence, which restores ACE2 binding and increases the interaction with convalescent plasma.
Organizational Affiliation:
School of Engineering Sciences in Chemistry, Biotechnology and Health, Department of Fibre and Polymer Technology, KTH Royal Institute of Technology, Stockholm, Sweden.