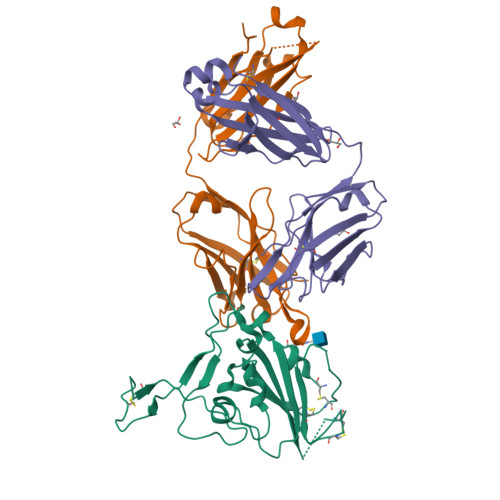
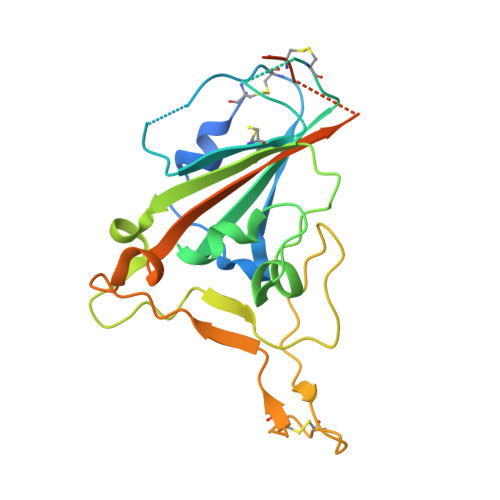
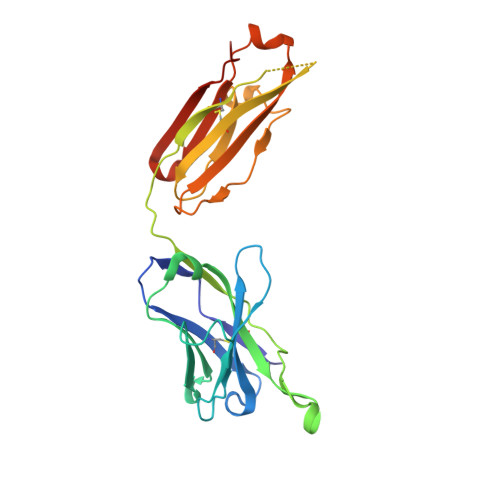
Efficacy of the combination of monoclonal antibodies against the SARS-CoV-2 Beta and Delta variants.
Boonkrai, C., Cotrone, T.S., Chaisuriyong, W., Tantawichien, T., Thisyakorn, U., Fernandez, S., Hunsawong, T., Reed, M., Wongtangprasert, T., Audomsun, T., Phakham, T., Attakitbancha, C., Saelao, P., Focht, D., Kimbung, R., Welin, M., Malik, A.A., Pisitkun, T., Srisawat, N.(2023) PLoS One 18: e0284173-e0284173
- PubMed: 37141227
- DOI: https://doi.org/10.1371/journal.pone.0284173
- Primary Citation of Related Structures:
8BSE, 8BSF - PubMed Abstract:
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is currently the biggest healthcare issue worldwide. This study aimed to develop a monoclonal antibody against SARS-CoV-2 from B cells of recovered COVID-19 patients, which might have beneficial therapeutic purposes for COVID-19 patients. We successfully generated human monoclonal antibodies (hmAbs) against the receptor binding domain (RBD) protein of SARS-CoV-2 using developed hybridoma technology. The isolated hmAbs against the RBD protein (wild-type) showed high binding activity and neutralized the interaction between the RBD and the cellular receptor angiotensin-converting enzyme 2 (ACE2) protein. Epitope binning and crystallography results displayed target epitopes of these antibodies in distinct regions beneficial in the mix as a cocktail. The 3D2 binds to conserved epitopes among multi-variants. Pseudovirion-based neutralization results revealed that the antibody cocktail, 1D1 and 3D2, showed high potency in multiple variants of SARS-CoV-2 infection. In vivo studies showed the ability of the antibody cocktail treatment (intraperitoneal (i.p.) administration) to reduce viral load (Beta variant) in blood and various tissues. While the antibody cocktail treatment (intranasal (i.n.) administration) could not significantly reduce the viral load in nasal turbinate and lung tissue, it could reduce the viral load in blood, kidney, and brain tissue. These findings revealed that the efficacy of the antibody cocktail, 1D1 and 3D2, should be further studied in animal models in terms of timing of administration, optimal dose, and efficacy to mitigate inflammation in targeted tissue such as nasal turbinate and lung.
Organizational Affiliation:
Interdisciplinary Program of Biomedical Sciences, Graduate School, Chulalongkorn University, Bangkok, Thailand.