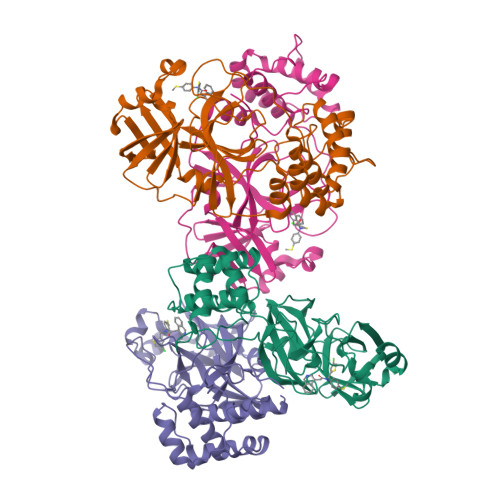
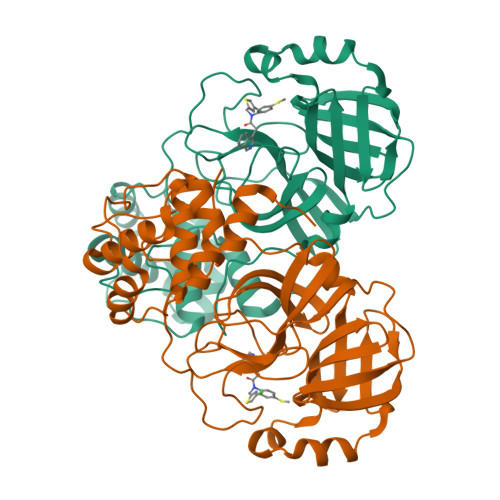
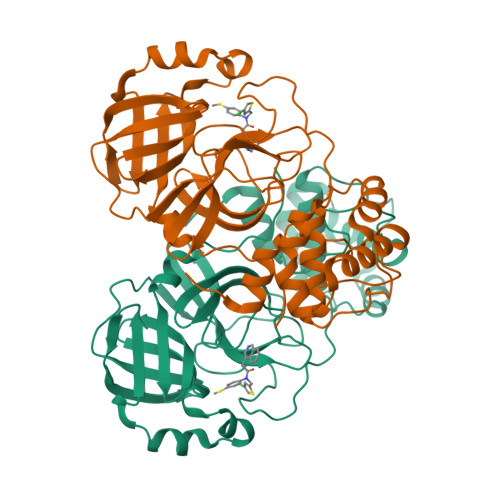
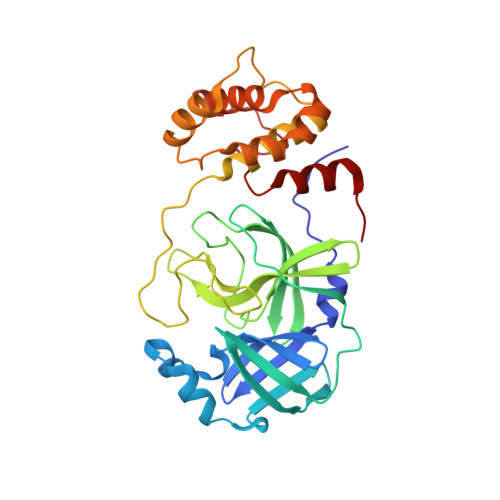
A novel class of broad-spectrum active-site-directed 3C-like protease inhibitors with nanomolar antiviral activity against highly immune-evasive SARS-CoV-2 Omicron subvariants.
Perez-Vargas, J., Worrall, L.J., Olmstead, A.D., Ton, A.T., Lee, J., Villanueva, I., Thompson, C.A.H., Dudek, S., Ennis, S., Smith, J.R., Shapira, T., De Guzman, J., Gang, S., Ban, F., Vuckovic, M., Bielecki, M., Kovacic, S., Kenward, C., Hong, C.Y., Gordon, D.G., Levett, P.N., Krajden, M., Leduc, R., Boudreault, P.L., Niikura, M., Paetzel, M., Young, R.N., Cherkasov, A., Strynadka, N.C.J., Jean, F.(2023) Emerg Microbes Infect 12: 2246594-2246594
- PubMed: 37555275
- DOI: https://doi.org/10.1080/22221751.2023.2246594
- Primary Citation of Related Structures:
8CYU, 8CYZ, 8CZ4, 8CZ7, 8SXR - PubMed Abstract:
Antivirals with broad coronavirus activity are important for treating high-risk individuals exposed to the constantly evolving SARS-CoV-2 variants of concern (VOCs) as well as emerging drug-resistant variants. We developed and characterized a novel class of active-site-directed 3-chymotrypsin-like protease (3CLpro) inhibitors ( C2-C5a ). Our lead direct-acting antiviral (DAA), C5a , is a non-covalent, non-peptide with a dissociation constant of 170 nM against recombinant SARS-CoV-2 3CLpro. The compounds C2-C5a exhibit broad-spectrum activity against Omicron subvariants (BA.5, BQ.1.1, and XBB.1.5) and seasonal human coronavirus-229E infection in human cells. Notably, C5a has median effective concentrations of 30-50 nM against BQ.1.1 and XBB.1.5 in two different human cell lines. X-ray crystallography has confirmed the unique binding modes of C2-C5a to the 3CLpro, which can limit virus cross-resistance to emerging Paxlovid-resistant variants. We tested the effect of C5a with two of our newly discovered host-directed antivirals (HDAs): N-0385, a TMPRSS2 inhibitor, and bafilomycin D (BafD), a human vacuolar H + -ATPase [V-ATPase] inhibitor. We demonstrated a synergistic action of C5a in combination with N-0385 and BafD against Omicron BA.5 infection in human Calu-3 lung cells. Our findings underscore that a SARS-CoV-2 multi-targeted treatment for circulating Omicron subvariants based on DAAs ( C5a ) and HDAs (N-0385 or BafD) can lead to therapeutic benefits by enhancing treatment efficacy. Furthermore, the high-resolution structures of SARS-CoV-2 3CLpro in complex with C2-C5a will facilitate future rational optimization of our novel broad-spectrum active-site-directed 3C-like protease inhibitors.
Organizational Affiliation:
Department of Microbiology and Immunology, Life Sciences Institute, University of British Columbia, Vancouver, Canada.