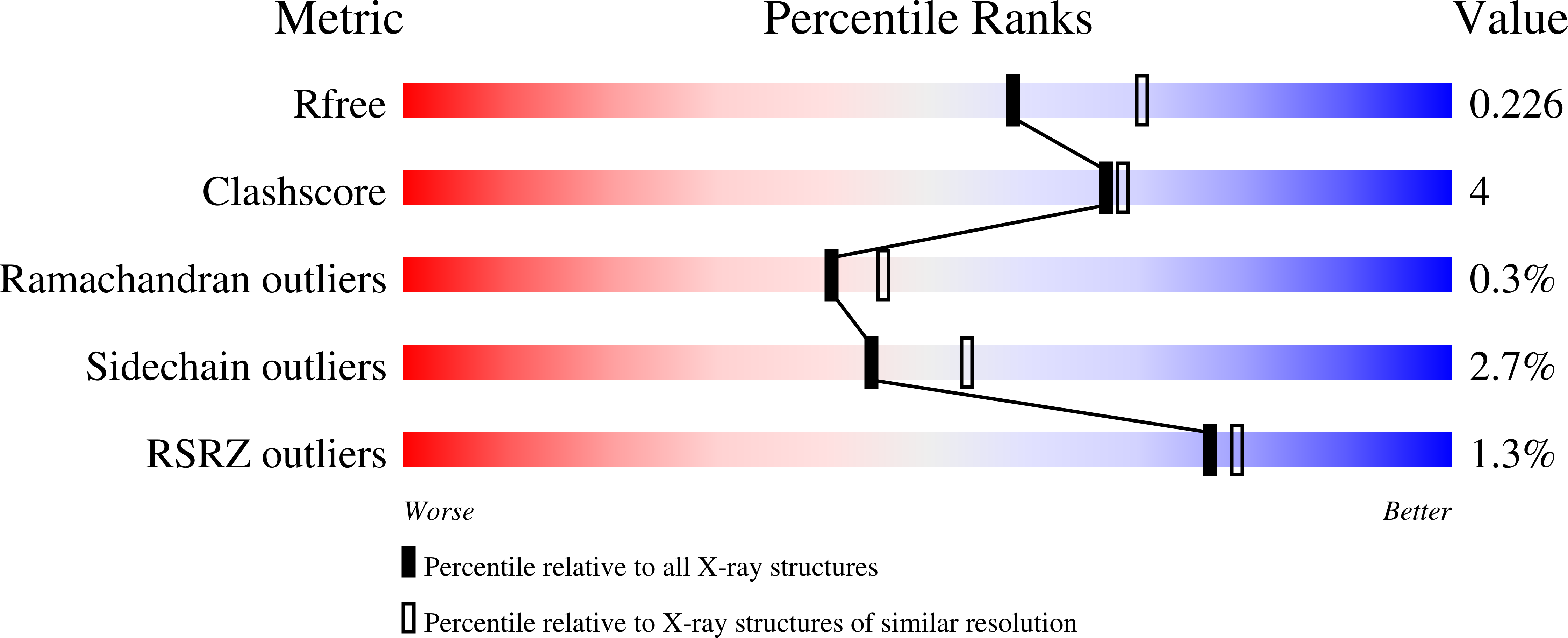
Structural basis of Qng1-mediated salvage of the micronutrient queuine from queuosine-5'-monophosphate as the biological substrate.
Hung, S.H., Elliott, G.I., Ramkumar, T.R., Burtnyak, L., McGrenaghan, C.J., Alkuzweny, S., Quaiyum, S., Iwata-Reuyl, D., Pan, X., Green, B.D., Kelly, V.P., de Crecy-Lagard, V., Swairjo, M.A.(2023) Nucleic Acids Res 51: 935-951
- PubMed: 36610787
- DOI: https://doi.org/10.1093/nar/gkac1231
- Primary Citation of Related Structures:
7U07, 7U1O, 7U5A, 7U91, 7UGK, 7UK3, 7ULC, 8DL3 - PubMed Abstract:
Eukaryotic life benefits from-and ofttimes critically relies upon-the de novo biosynthesis and supply of vitamins and micronutrients from bacteria. The micronutrient queuosine (Q), derived from diet and/or the gut microbiome, is used as a source of the nucleobase queuine, which once incorporated into the anticodon of tRNA contributes to translational efficiency and accuracy. Here, we report high-resolution, substrate-bound crystal structures of the Sphaerobacter thermophilus queuine salvage protein Qng1 (formerly DUF2419) and of its human ortholog QNG1 (C9orf64), which together with biochemical and genetic evidence demonstrate its function as the hydrolase releasing queuine from queuosine-5'-monophosphate as the biological substrate. We also show that QNG1 is highly expressed in the liver, with implications for Q salvage and recycling. The essential role of this family of hydrolases in supplying queuine in eukaryotes places it at the nexus of numerous (patho)physiological processes associated with queuine deficiency, including altered metabolism, proliferation, differentiation and cancer progression.
Organizational Affiliation:
Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA.