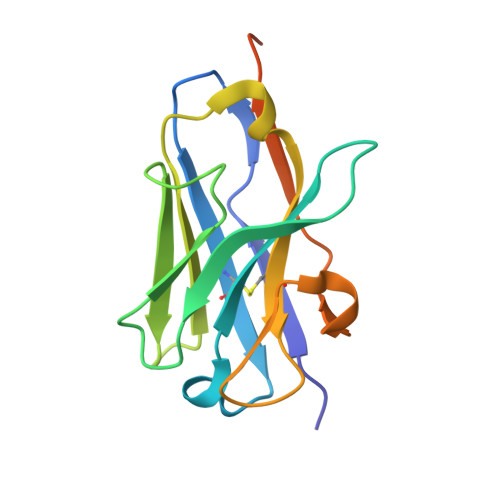
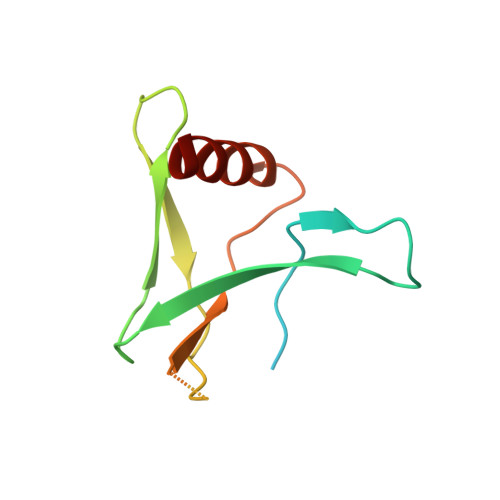
Inside-out: Antibody-binding reveals potential folding hinge-points within the SARS-CoV-2 replication co-factor nsp9.
Pan, Y., Chandrashekaran, I.R., Tennant, L., Rossjohn, J., Littler, D.R.(2023) PLoS One 18: e0283194-e0283194
- PubMed: 37036856
- DOI: https://doi.org/10.1371/journal.pone.0283194
- Primary Citation of Related Structures:
8DQU - PubMed Abstract:
Nsp9 is a conserved accessory component of the coronaviral replication and transcription complex. It is the predominant substrate of nsp12's nucleotidylation activity while also serving to recruit proteins required for viral 5'-capping. Anti-nsp9 specific nanobodies have been isolated previously. We confirm that their binding mode is centred upon Trp-53 within SARS-CoV-2 nsp9. Antibody binding at this site surprisingly results in large-scale changes to the overall topology of this coronaviral unique fold. We further characterise the antibody-induced structural dynamism within nsp9, identifying a number of potentially flexible regions. A large expansion of the cavity between the s2-s3 and s4-s5 loops is particularly noteworthy. As is the potential for large-scale movements in the C-terminal GxxxG helix.
Organizational Affiliation:
Monash Institute of Pharmaceutical Sciences, Monash University, Parkville, Victoria, Australia.