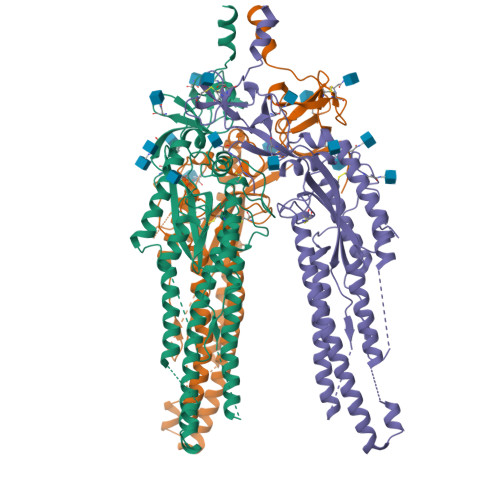
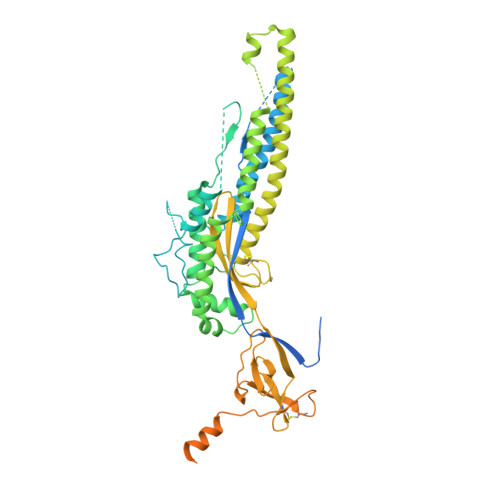
SARS-CoV-2 spike conformation determines plasma neutralizing activity elicited by a wide panel of human vaccines.
Bowen, J.E., Park, Y.J., Stewart, C., Brown, J.T., Sharkey, W.K., Walls, A.C., Joshi, A., Sprouse, K.R., McCallum, M., Tortorici, M.A., Franko, N.M., Logue, J.K., Mazzitelli, I.G., Nguyen, A.W., Silva, R.P., Huang, Y., Low, J.S., Jerak, J., Tiles, S.W., Ahmed, K., Shariq, A., Dan, J.M., Zhang, Z., Weiskopf, D., Sette, A., Snell, G., Posavad, C.M., Iqbal, N.T., Geffner, J., Bandera, A., Gori, A., Sallusto, F., Maynard, J.A., Crotty, S., Van Voorhis, W.C., Simmerling, C., Grifantini, R., Chu, H.Y., Corti, D., Veesler, D.(2022) Sci Immunol 7: eadf1421-eadf1421
- PubMed: 36356052
- DOI: https://doi.org/10.1126/sciimmunol.adf1421
- Primary Citation of Related Structures:
8DYA - PubMed Abstract:
Numerous safe and effective coronavirus disease 2019 vaccines have been developed worldwide that use various delivery technologies and engineering strategies. We show here that vaccines containing prefusion-stabilizing S mutations elicit antibody responses in humans with enhanced recognition of S and the S 1 subunit relative to postfusion S as compared with vaccines lacking these mutations or natural infection. Prefusion S and S 1 antibody binding titers positively and equivalently correlated with neutralizing activity, and depletion of S 1 -directed antibodies completely abrogated plasma neutralizing activity. We show that neutralizing activity is almost entirely directed to the S 1 subunit and that variant cross-neutralization is mediated solely by receptor binding domain-specific antibodies. Our data provide a quantitative framework for guiding future S engineering efforts to develop vaccines with higher resilience to the emergence of variants than current technologies.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, WA 98195, USA.