Development of the Safe and Broad-Spectrum Aldehyde and Ketoamide Mpro inhibitors Derived from the Constrained alpha , gamma-AA Peptide Scaffold.
Wang, L., Ma, C., Sacco, M.D., Xue, S., Mahmoud, M., Calcul, L., Chen, Y., Wang, J., Cai, J.(2023) Chemistry 29: e202300476-e202300476
- PubMed: 36920943
- DOI: https://doi.org/10.1002/chem.202300476
- Primary Citation of Related Structures:
8DZB, 8DZC - PubMed Abstract:
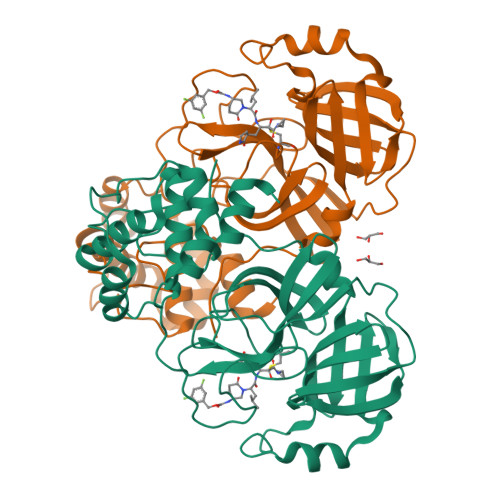
SARS-CoV-2 is still wreaking havoc all over the world with surging morbidity and high mortality. The main protease (M pro ) is essential in the replication of SARS-CoV-2, enabling itself an active target for antiviral development. Herein, we reported the design and synthesis of a new class of peptidomimetics-constrained α, γ-AA peptides, based on which a series of aldehyde and ketoamide inhibitors of the M pro of SARS-CoV-2 were prepared. The lead compounds showed excellent inhibitory activity in the FRET-based M pro enzymatic assay not only for the M pro of SARS-CoV-2 but also for SARS-CoV and MERS-CoV, along with HCoVs like HCoV-OC43, HCoV-229E, HCoV-NL63 and HKU1. The X-ray crystallographic results demonstrated that our compounds form a covalent bond with the catalytic Cys145. They also demonstrated effective antiviral activity against live SARS-CoV-2. Overall, the results suggest that α, γ-AA peptide could be a promising molecular scaffold in designing novel M pro inhibitors of SARS-CoV-2 and other coronaviruses.
Organizational Affiliation:
Department of Chemistry, University of South Florida, 4202 E. Fowler Ave., Tampa, FL 33620, USA.