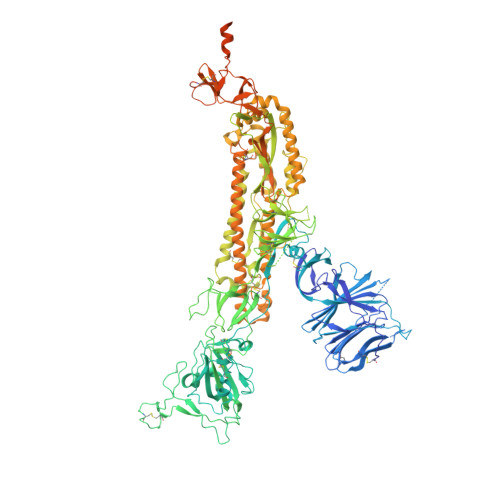
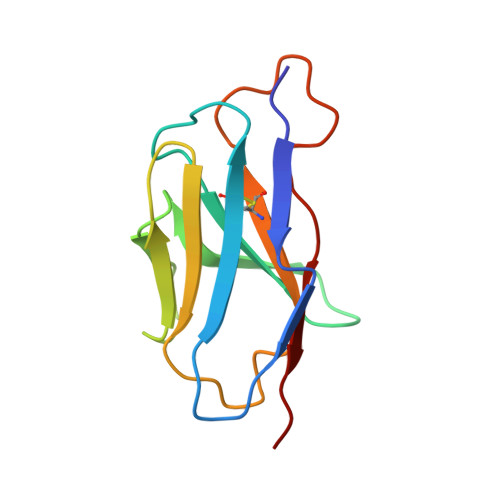
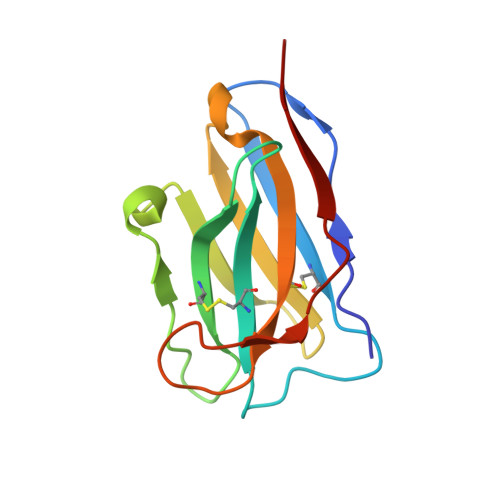
Mechanism of a rabbit monoclonal antibody broadly neutralizing SARS-CoV-2 variants.
Guo, H., Yang, Y., Zhao, T., Lu, Y., Gao, Y., Li, T., Xiao, H., Chu, X., Zheng, L., Li, W., Cheng, H., Huang, H., Liu, Y., Lou, Y., Nguyen, H.C., Wu, C., Chen, Y., Yang, H., Ji, X.(2023) Commun Biol 6: 364-364
- PubMed: 37012333
- DOI: https://doi.org/10.1038/s42003-023-04759-5
- Primary Citation of Related Structures:
8GZZ, 8H00, 8H01, 8ITU - PubMed Abstract:
Due to the continuous evolution of SARS-CoV-2, the Omicron variant has emerged and exhibits severe immune evasion. The high number of mutations at key antigenic sites on the spike protein has made a large number of existing antibodies and vaccines ineffective against this variant. Therefore, it is urgent to develop efficient broad-spectrum neutralizing therapeutic drugs. Here we characterize a rabbit monoclonal antibody (RmAb) 1H1 with broad-spectrum neutralizing potency against Omicron sublineages including BA.1, BA.1.1, BA.2, BA.2.12.1, BA.2.75, BA.3 and BA.4/5. Cryo-electron microscopy (cryo-EM) structure determination of the BA.1 spike-1H1 Fab complexes shows that 1H1 targets a highly conserved region of RBD and avoids most of the circulating Omicron mutations, explaining its broad-spectrum neutralization potency. Our findings indicate 1H1 as a promising RmAb model for designing broad-spectrum neutralizing antibodies and shed light on the development of therapeutic agents as well as effective vaccines against newly emerging variants in the future.
Organizational Affiliation:
The State Key Laboratory of Pharmaceutical Biotechnology, School of Life Sciences, Nanjing University, Nanjing, Jiangsu, 210023, China.