Structural and Biological Evaluations of a Non-Nucleoside STING Agonist Specific for Human STING A230 Variants.
Tang, Z., Zhao, J., Li, Y., Tomer, S., Selvaraju, M., Tien, N., Sun, D., Johnson, D.K., Zhen, A., Li, P., Wang, J.(2023) bioRxiv
- PubMed: 37425806
- DOI: https://doi.org/10.1101/2023.07.02.547363
- Primary Citation of Related Structures:
8T5K, 8T5L - PubMed Abstract:
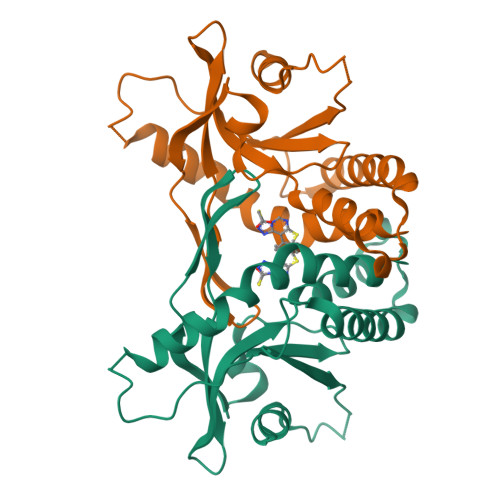
Previously we identified a non-nucleotide tricyclic agonist BDW568 that activates human STING (stimulator of interferon genes) gene variant containing A230 in a human monocyte cell line (THP-1). STING A230 alleles, including HAQ and AQ, are less common STING variants in human population. To further characterize the mechanism of BDW568, we obtained the crystal structure of the C-terminal domain of STING A230 complexed with BDW-OH (active metabolite of BDW568) at 1.95 Å resolution and found the planar tricyclic structure in BDW-OH dimerizes in the STING binding pocket and mimics the two nucleobases of the endogenous STING ligand 2',3'-cGAMP. This binding mode also resembles a known synthetic ligand of human STING, MSA-2, but not another tricyclic mouse STING agonist DMXAA. Structure-activity-relationship (SAR) studies revealed that all three heterocycles in BDW568 and the S-acetate side chain are critical for retaining the compound's activity. BDW568 could robustly activate the STING pathway in human primary peripheral blood mononuclear cells (PBMCs) with STING A230 genotype from healthy individuals. We also observed BDW568 could robustly activate type I interferon signaling in purified human primary macrophages that were transduced with lentivirus expressing STING A230 , suggesting its potential use to selectively activate genetically engineered macrophages in macrophage-based approaches, such as chimeric antigen receptor (CAR)-macrophage immunotherapies.
Organizational Affiliation:
Department of Medicinal Chemistry, University of Kansas, Lawrence, Kansas 66047, United States.