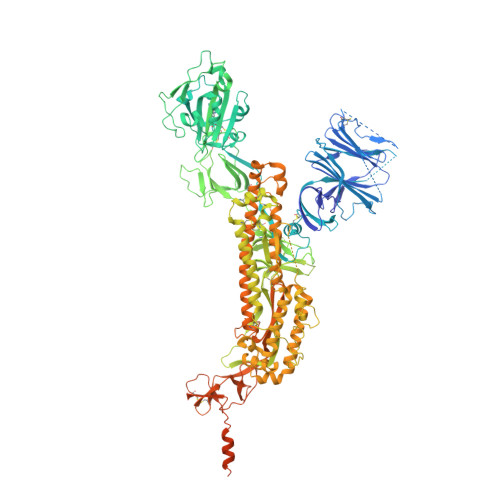
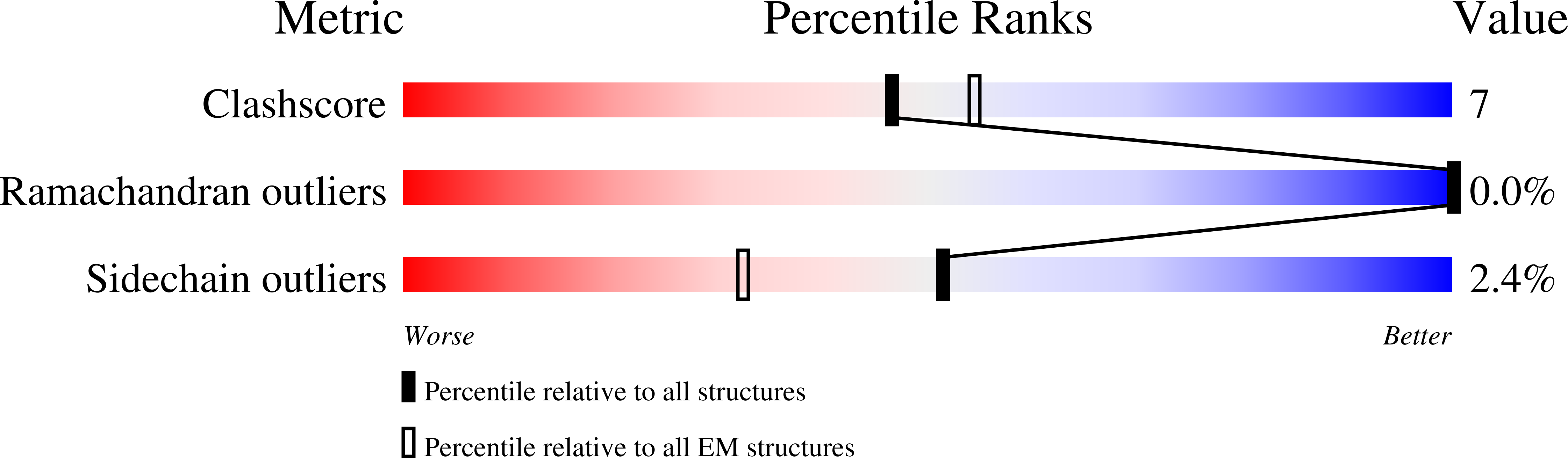Improving resolution and resolvability of single-particle cryoEM structures using Gaussian mixture models.
Chen, M., Schmid, M.F., Chiu, W.(2024) Nat Methods 21: 37-40
- PubMed: 37973972
- DOI: https://doi.org/10.1038/s41592-023-02082-9
- Primary Citation of Related Structures:
8U26, 8U28, 8U2C - PubMed Abstract:
Cryogenic electron microscopy is widely used in structural biology, but its resolution is often limited by the dynamics of the macromolecule. Here we developed a refinement protocol based on Gaussian mixture models that integrates particle orientation and conformation estimation and improves the alignment for flexible domains of protein structures. We demonstrated this protocol on multiple datasets, resulting in improved resolution and resolvability, locally and globally, by visual and quantitative measures.
Organizational Affiliation:
Division of CryoEM and Bioimaging, SSRL, SLAC National Accelerator Laboratory, Stanford University, Menlo Park, CA, USA. muyuanc@stanford.edu.