Structural and Functional Characterization of a Novel Class A Flavin Monooxygenase from Bacillus niacini.
Richardson, B.C., Turlington, Z.R., Vaz Ferreira de Macedo, S., Phillips, S.K., Perry, K., Brancato, S.G., Cooke, E.W., Gwilt, J.R., Dasovich, M.A., Roering, A.J., Rossi, F.M., Snider, M.J., French, J.B., Hicks, K.A.(2024) Biochemistry 63: 2506-2516
- PubMed: 39265075
- DOI: https://doi.org/10.1021/acs.biochem.4c00306
- Primary Citation of Related Structures:
8UIU, 8URC, 8URD - PubMed Abstract:
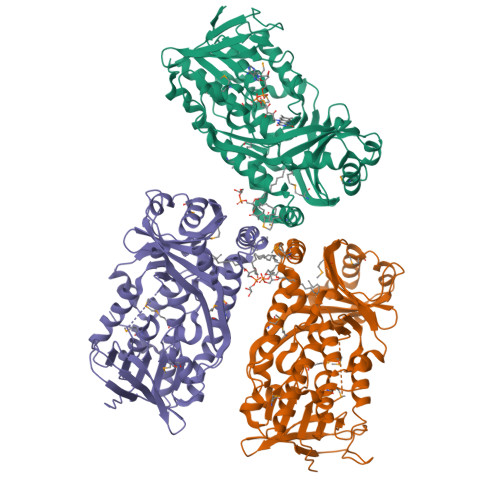
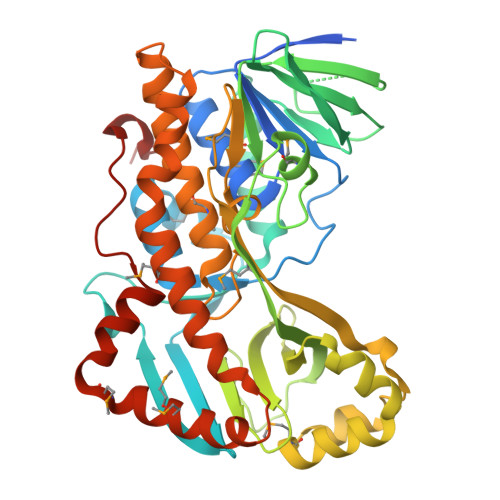
A gene cluster responsible for the degradation of nicotinic acid (NA) in Bacillus niacini has recently been identified, and the structures and functions of the resulting enzymes are currently being evaluated to establish pathway intermediates. One of the genes within this cluster encodes a flavin monooxygenase (BnFMO) that is hypothesized to catalyze a hydroxylation reaction. Kinetic analyses of the recombinantly purified BnFMO suggest that this enzyme catalyzes the hydroxylation of 2,6-dihydroxynicotinic acid (2,6-DHNA) or 2,6-dihydroxypyridine (2,6-DHP), which is formed spontaneously by the decarboxylation of 2,6-DHNA. To understand the details of this hydroxylation reaction, we determined the structure of BnFMO using a multimodel approach combining protein X-ray crystallography and cryo-electron microscopy (cryo-EM). A liganded BnFMO cryo-EM structure was obtained in the presence of 2,6-DHP, allowing us to make predictions about potential catalytic residues. The structural data demonstrate that BnFMO is trimeric, which is unusual for Class A flavin monooxygenases. In both the electron density and coulomb potential maps, a region at the trimeric interface was observed that was consistent with and modeled as lipid molecules. High-resolution mass spectral analysis suggests that there is a mixture of phosphatidylethanolamine and phosphatidylglycerol lipids present. Together, these data provide insights into the molecular details of the central hydroxylation reaction unique to the aerobic degradation of NA in Bacillus niacini .
Organizational Affiliation:
The Hormel Institute, University of Minnesota, Austin, Minnesota 55912, United States.