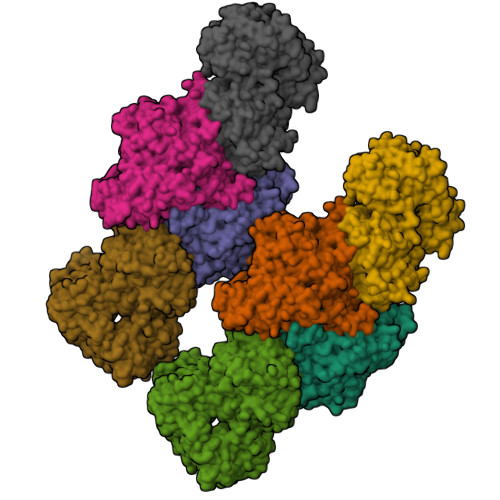
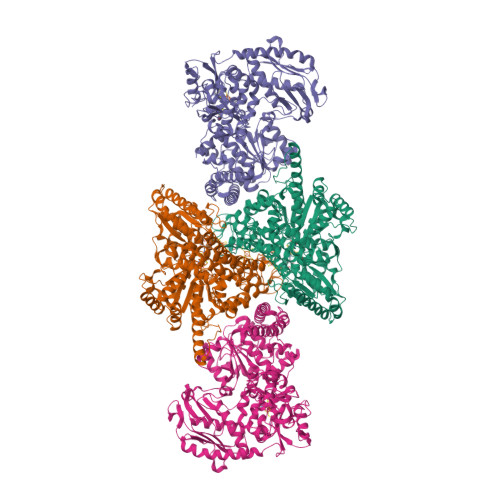
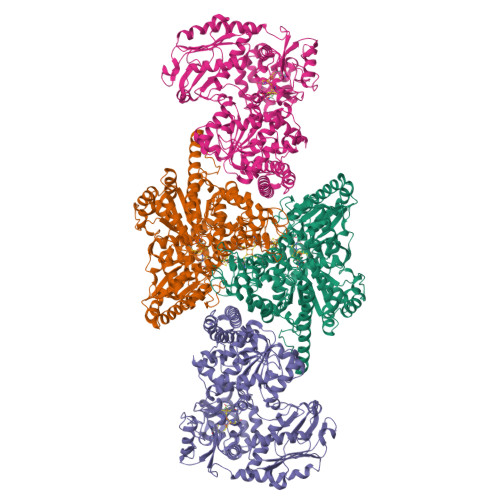
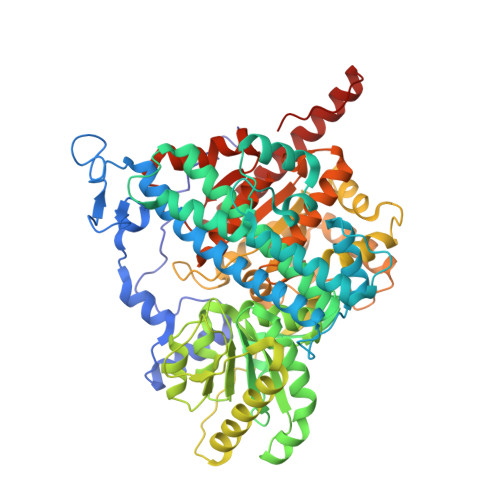
A Ni-Fe-Cu center in a bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase
Doukov, T.I., Iverson, T.M., Seravalli, J., Ragsdale, S.W., Drennan, C.L.(2002) Science 298: 567-572
- PubMed: 12386327
- DOI: https://doi.org/10.1126/science.1075843
- Primary Citation of Related Structures:
1MJG - PubMed Abstract:
A metallocofactor containing iron, sulfur, copper, and nickel has been discovered in the enzyme carbon monoxide dehydrogenase/acetyl-CoA (coenzyme A) synthase from Moorella thermoacetica (f. Clostridium thermoaceticum). Our structure at 2.2 angstrom resolution reveals that the cofactor responsible for the assembly of acetyl-CoA contains a [Fe4S4] cubane bridged to a copper-nickel binuclear site. The presence of these three metals together in one cluster was unanticipated and suggests a newly discovered role for copper in biology. The different active sites of this bifunctional enzyme complex are connected via a channel, 138 angstroms long, that provides a conduit for carbon monoxide generated at the C-cluster on one subunit to be incorporated into acetyl-CoA at the A-cluster on the other subunit.
Organizational Affiliation:
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.