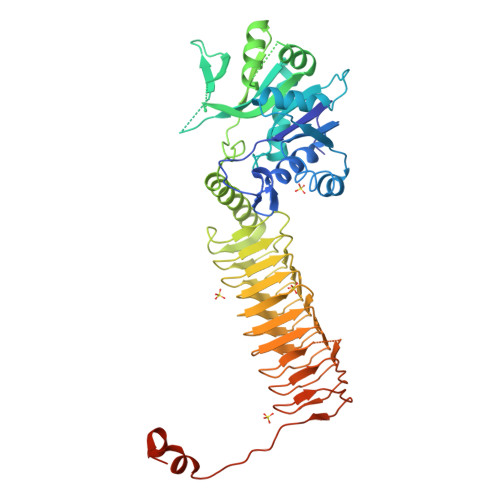
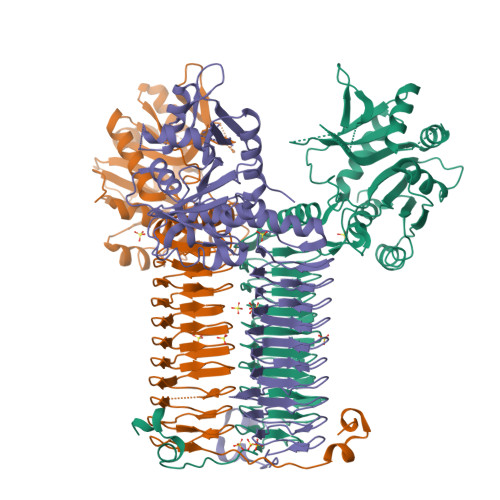
Structure of N-acetylglucosamine-1-phosphate uridyltransferase (GlmU) from Mycobacterium tuberculosis in a cubic space group.
Verma, S.K., Jaiswal, M., Kumar, N., Parikh, A., Nandicoori, V.K., Prakash, B.(2009) Acta Crystallogr Sect F Struct Biol Cryst Commun 65: 435-439
- PubMed: 19407371
- DOI: https://doi.org/10.1107/S1744309109010252
- Primary Citation of Related Structures:
3FOQ - PubMed Abstract:
GlmU is a bifunctional enzyme that catalyzes the final two steps in the biosynthesis of UDP-GlcNAc. Crystals of GlmU from Mycobacterium tuberculosis obtained using ammonium sulfate as a precipitant diffracted poorly (to 3.4 A resolution) and displayed an unusually high solvent content (>80%) with sparse crystal packing that resulted in large solvent channels. With one molecule per asymmetric unit, the monomers from three neighbouring asymmetric units related by the crystal threefold formed a biological trimer. Although this is the first report of the structure of GlmU determined in a cubic crystal form, the trimeric arrangement here is similar to that observed for other GlmU structures determined in hexagonal (H3, H32, P6(3)22) space groups.
Organizational Affiliation:
Department of Biological Sciences and Bioengineering, Indian Institute of Technology, Kanpur, India.