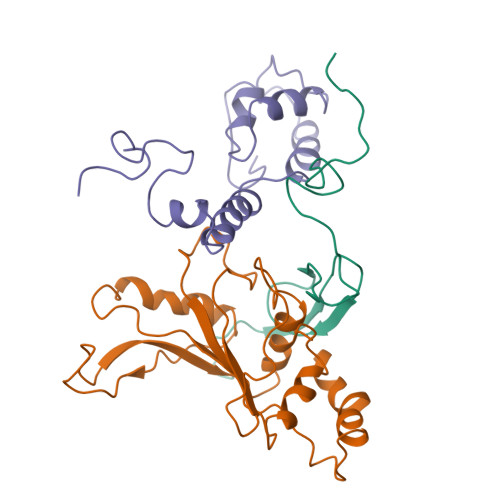
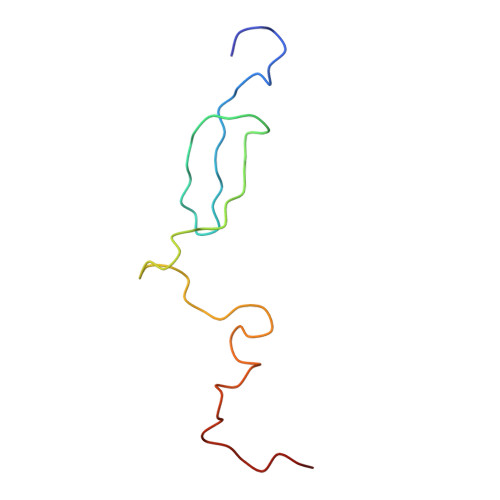
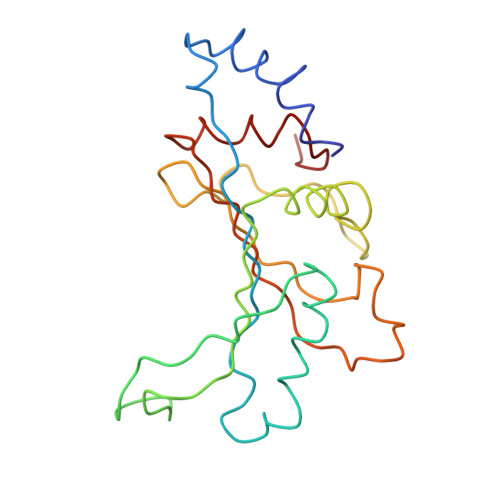
Intrinsic molecular properties of the protein-protein bridge facilitate ratchet-like motion of the ribosome.
Shasmal, M., Chakraborty, B., Sengupta, J.(2010) Biochem Biophys Res Commun 399: 192-197
- PubMed: 20643101
- DOI: https://doi.org/10.1016/j.bbrc.2010.07.053
- Primary Citation of Related Structures:
3IYX, 3IYY - PubMed Abstract:
The ribosomal intersubunit bridges maintain the overall architecture of the ribosome and thereby play a pivotal role in the dynamics of translation. The only protein-protein bridge, b1b, is formed by the two proteins, S13 and L5 of the small and large ribosomal subunits, respectively. B1b absorbs the largest movement during ratchet-like motion, and its two proteins reorganize in different constellations during this motion of the ribosome. Our results in this study of b1b in the Escherichia coli 70S ribosome suggest that the intrinsic molecular features of the bridging proteins allow the bridge to modulate the ratchet-like motion in a controlled manner. Additionally, another large subunit protein, L31, seems to participate with S13 and L5 in the formation, dynamics, and stabilization of this bridge.
Organizational Affiliation:
Structural Biology and Bioinformatics Division, Indian Institute of Chemical Biology, 4 Raja S.C. Mullick Road, Calcutta 700032, India.