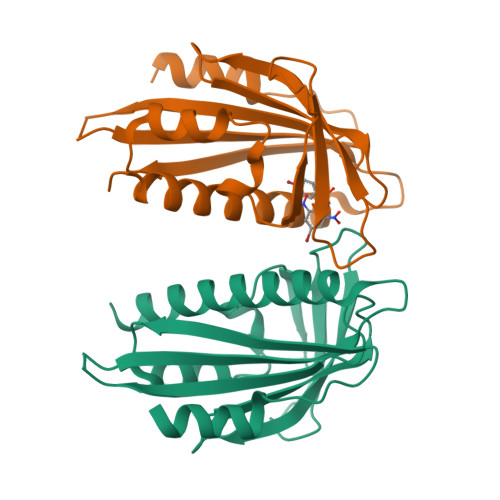
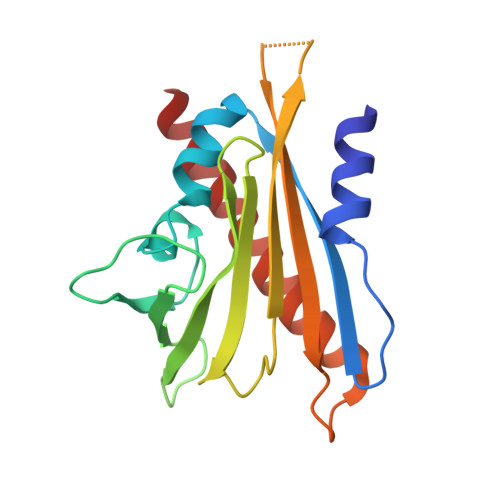
Stabilization of dimeric PYR/PYL/RCAR family members relieves abscisic acid-induced inhibition of seed germination.
Wang, Z.Z., Cao, M.J., Yan, J., Dong, J., Chen, M.X., Yang, J.F., Li, J.H., Ying, R.N., Gao, Y.Y., Li, L., Leng, Y.N., Tian, Y., Hewage, K.A.H., Pei, R.J., Huang, Z.Y., Yin, P., Zhu, J.K., Hao, G.F., Yang, G.F.(2024) Nat Commun 15: 8077-8077
- PubMed: 39277642
- DOI: https://doi.org/10.1038/s41467-024-52426-y
- Primary Citation of Related Structures:
9J6I - PubMed Abstract:
Abscisic acid (ABA) is the primary preventing factor of seed germination, which is crucial to plant survival and propagation. ABA-induced seed germination inhibition is mainly mediated by the dimeric PYR/PYL/RCAR (PYLs) family members. However, little is known about the relevance between dimeric stability of PYLs and seed germination. Here, we reveal that stabilization of PYL dimer can relieve ABA-induced inhibition of seed germination using chemical genetic approaches. Di-nitrobensulfamide (DBSA), a computationally designed chemical probe, yields around ten-fold improvement in receptor affinity relative to ABA. DBSA reverses ABA-induced inhibition of seed germination mainly through dimeric receptors and recovers the expression of ABA-responsive genes. DBSA maintains PYR1 in dimeric state during protein oligomeric state experiment. X-ray crystallography shows that DBSA targets a pocket in PYL dimer interface and may stabilize PYL dimer by forming hydrogen networks. Our results illustrate the potential of PYL dimer stabilization in preventing ABA-induced seed germination inhibition.
Organizational Affiliation:
State Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, Central China Normal University, Wuhan, 430079, China.