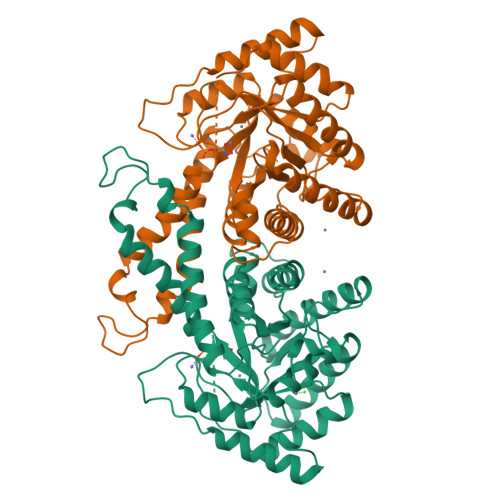
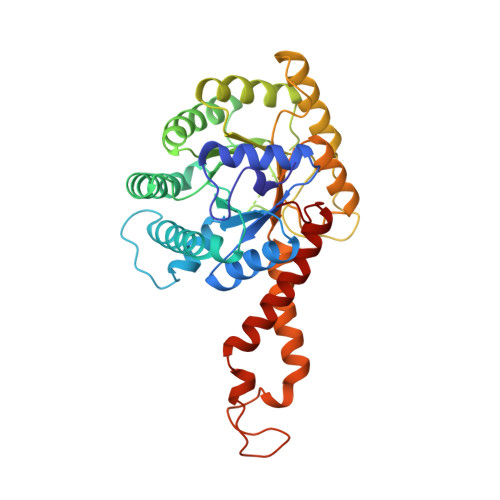
The crystal structure of Escherichia coli class II fructose-1, 6-bisphosphate aldolase in complex with phosphoglycolohydroxamate reveals details of mechanism and specificity.
Hall, D.R., Leonard, G.A., Reed, C.D., Watt, C.I., Berry, A., Hunter, W.N.(1999) J Mol Biology 287: 383-394
- PubMed: 10080900
- DOI: https://doi.org/10.1006/jmbi.1999.2609
- Primary Citation of Related Structures:
1B57 - PubMed Abstract:
The structure of a class II fructose-1,6-bisphosphate aldolase in complex with the substrate analogue and inhibitor phosphoglycolohydroxamate (PGH) has been determined using X-ray diffraction terms to a resolution of 2.0 A (1 A=0.1 nm). The crystals are trigonal, space group P3121 with a=b=78.24 A, c=289.69 A. The asymmetric unit is a homodimer of (alpha/beta)8 barrels and the model has refined to give R-work 19.2 %, R-free (based on 5 % of the data) 23.0 %. PGH resembles the ene-diolate transition state of the physiological substrate dihydroxyacetone phosphate. It is well ordered and bound in a deep polar cavity at the C-terminal end of the (alpha/beta)8 barrel, where it chelates the catalytic zinc ion using hydroxyl and enolate oxygen atoms. Trigonal bipyramidal coordination of the zinc ion is completed by three histidine residues. The complex network of hydrogen bonds at the catalytic centre is required to organise the position of key functional groups and metal ion ligands. A well-defined monovalent cation-binding site is observed following significant re-organisation of loop structures. This assists the formation of a phosphate-binding site on one side of the barrel that tethers PGH in the catalytic site. The positions of functional groups of substrate and putative interactions with key amino acid residues are identified. Knowledge of the complex structure complements the results of spectroscopic and site-directed mutagenesis studies, and contributes to our understanding of the mechanism and substrate specificity of this family of enzymes. A reaction mechanism distinct from that proposed for other class II aldolases is discussed. The results suggest that the class II aldolases should be sub-divided into two groups on the basis of both distinct folds and mechanism.
Organizational Affiliation:
Department of Biochemistry, University of Dundee, Dundee, DD1 5EH, UK.