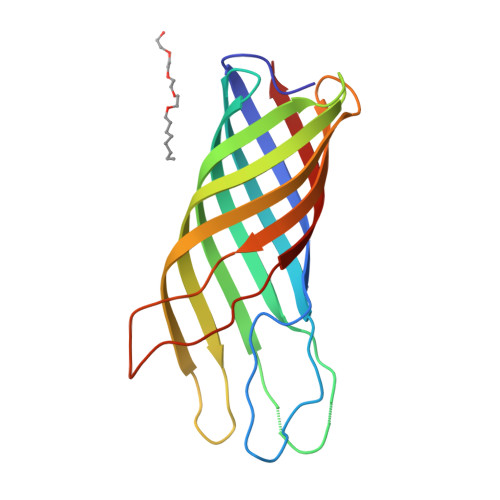
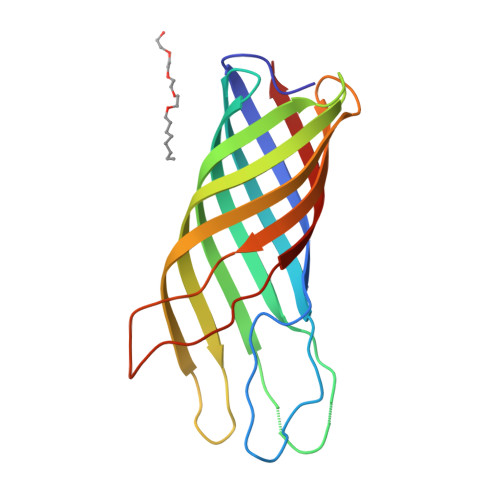
Structure of the outer membrane protein A transmembrane domain.
Pautsch, A., Schulz, G.E.(1998) Nat Struct Biol 5: 1013-1017
- PubMed: 9808047
- DOI: https://doi.org/10.1038/2983
- Primary Citation of Related Structures:
1BXW - PubMed Abstract:
The outer membrane protein A of Escherichia coli (OmpA) is an intensely studied example in the field of membrane protein folding. We have determined the structure of the OmpA transmembrane domain consisting of residues 1-171, by X-ray diffraction analysis, to a resolution of 2.5 A. It consists of a regular, extended eight-stranded beta-barrel and appears to be constructed like an inverse micelle with large water-filled cavities, but does not form a pore. Surprisingly, the cavities seem to be highly conserved during evolution. The structure corroborates the concept that all outer membrane proteins consist of beta-barrels. The structure constitutes a beta-barrel membrane anchor that appears to be the outer membrane equivalent of the single-chain alpha-helix anchor of the inner membrane.
Organizational Affiliation:
Institut für Organische Chemie und Biochemie, Albert-Ludwigs-Universität, Freiburg im Breisgau, Germany.