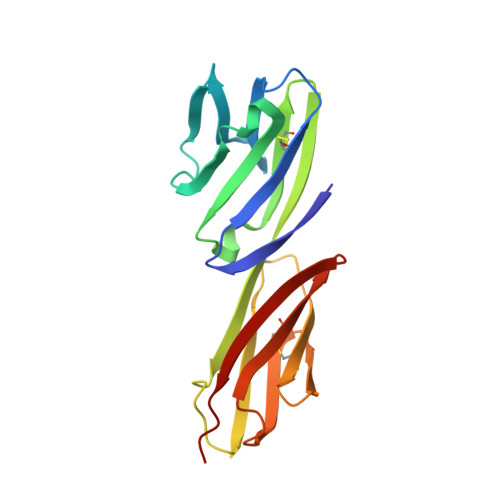
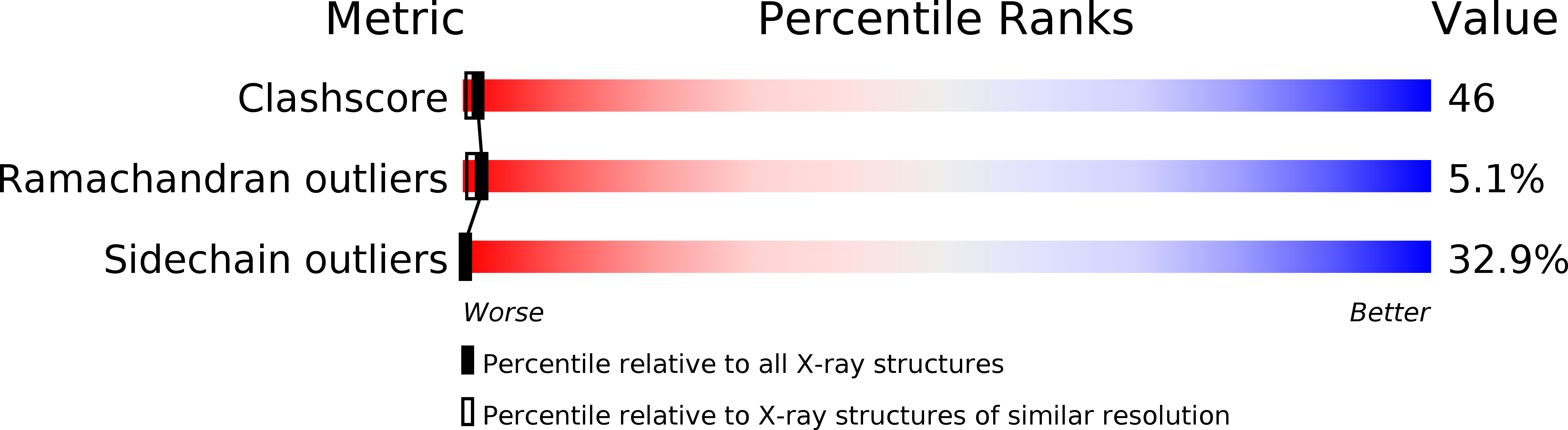Structures of an HIV and MHC binding fragment from human CD4 as refined in two crystal lattices.
Ryu, S.E., Truneh, A., Sweet, R.W., Hendrickson, W.A.(1994) Structure 2: 59-74
- PubMed: 8075984
- DOI: https://doi.org/10.1016/S0969-2126(00)00008-3
- Primary Citation of Related Structures:
1CDH, 1CDI - PubMed Abstract:
The T-cell surface glycoprotein CD4 interacts with class II molecules of the major histocompatibility complex (MHC) enhancing the signal for T-cell activation. Human CD4 also interacts, at high affinity, with the HIV envelope glycoprotein, gp120, to mediate T-cell infection by HIV. Crystal structures of amino-terminal two-domain (D1D2) fragments of human CD4, which contain the residues implicated in HIV and MHC interactions, have been reported earlier. We have determined the crystal structure of a new D1D2 construct by molecular replacement from a previously described crystal structure of D1D2. This structure has more uniform lattice contacts than are in the first. This gives an improved image of domain D2, which in turn has permitted further refinement of the initial structure at 2.3 A resolution against a more complete data set. The structure of the second crystal form was also refined at 2.9 A resolution. In both models, all residues from 1 to 178 are now well defined, including the loop regions in D2. Similarities of the molecular structure in the two lattices suggest that the D1D2 fragment works as a unit, with segmental flexibility largely restricted to the junction between domains D2 and D3. Variability of conformation in loops, including those implicated in MHC and HIV binding, requires an 'induced fit' in these interactions. Well defined density for the exposed side chain of Phe43 in both crystals confirms a prominent role for this residue in gp120 binding.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 10032.