High precision NMR structure of YhhP, a novel Escherichia coli protein implicated in cell division.
Katoh, E., Hatta, T., Shindo, H., Ishii, Y., Yamada, H., Mizuno, T., Yamazaki, T.(2000) J Mol Biology 304: 219-229
- PubMed: 11080457
- DOI: https://doi.org/10.1006/jmbi.2000.4170
- Primary Citation of Related Structures:
1DCJ - PubMed Abstract:
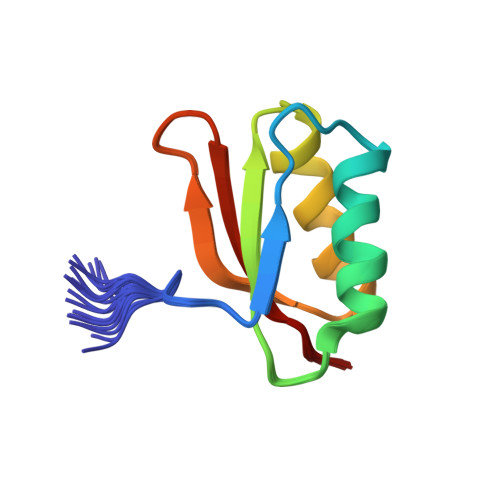
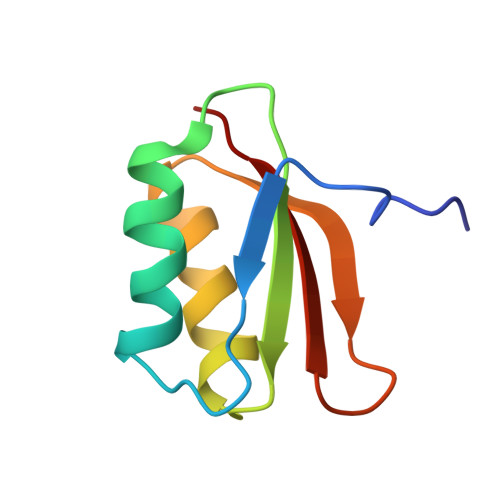
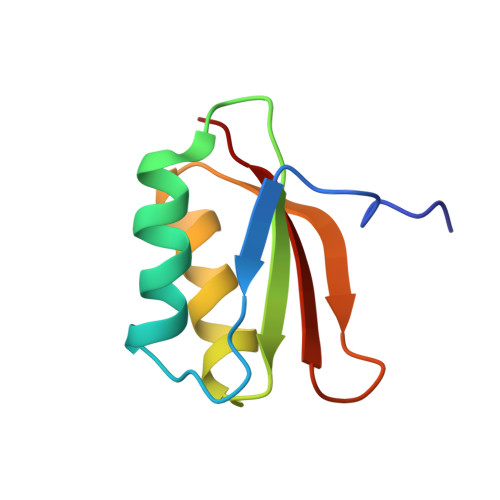
YhhP, a small protein of 81 amino acid residues encoded by the yhhP gene in the Escherichia coli database, is implicated in cell division although the precise biological function of this protein has not been yet identified. A variety of microorganisms have similar proteins, all of which contain a common CPxP sequence motif in the N-terminal region. We have determined the three-dimensional solution structure of YhhP by NMR spectroscopy in order to obtain insight into its biological function. It folds into a two-layered alpha/beta-sandwich structure with a betaalphabetaalphabetabeta fold, comprising a mixed four-stranded beta-sheet stacked against two alpha-helices, both of which are nearly parallel to the strands of the beta-sheet. The CPxP motif plays a significant structural role in stabilizing the first helix as a part of the new type N-capping box where the Cys-Pro peptide bond adopts a cis configuration. The structure of YhhP displays a striking resemblance to the C-terminal ribosome-binding domain of translation initiation factor IF3 (IF3C). In addition, the surface charge distribution of the RNA-recognition helix of IF3C is nearly the same as that of the corresponding helix of YhhP. These results suggest a structure-based hypothesis in which binding to an RNA target plays an essential role in the function of this ubiquitous protein.
Organizational Affiliation:
Structural Biology Unit, National Institute of Agrobiological Resources, Tsukuba, Ibaraki, 305-8602, Japan.