Crystal Structure of Hydroxynitrile Lyase from Sorghum Bicolor in Complex with the Inhibitor Benzoic Acid: A Novel Cyanogenic Enzyme
Lauble, H., Miehlich, B., Foerster, S., Wajant, H., Effenberger, F.(2002) Biochemistry 41: 12043
- PubMed: 12356304
- DOI: https://doi.org/10.1021/bi020300o
- Primary Citation of Related Structures:
1GXS - PubMed Abstract:
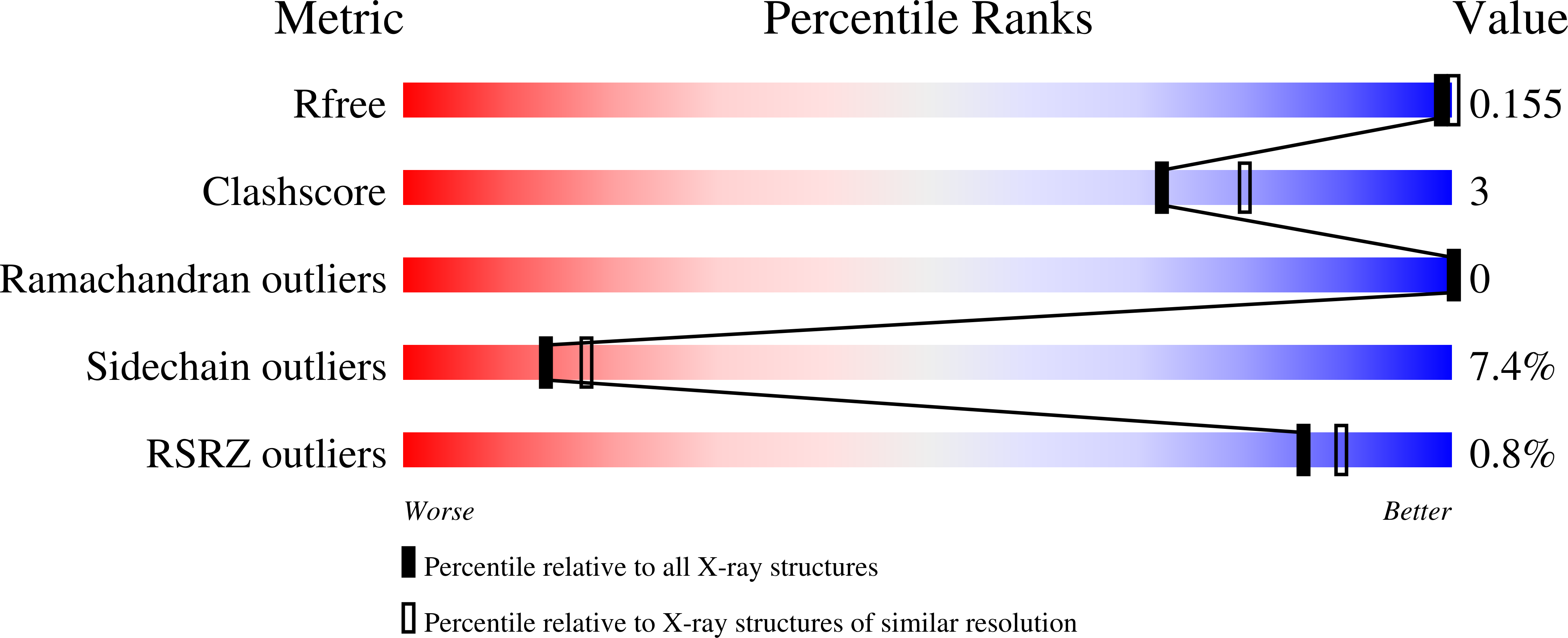
The crystal structure of the hydroxynitrile lyase from Sorghum bicolor (SbHNL) in complex with the inhibitor benzoic acid has been determined at 2.3 A resolution and refined to a crystallographic R-factor of 16.5%. The SbHNL sequence places the enzyme in the alpha/beta hydrolase family where the active site nucleophile is predicted to be organized in a characteristic pentapeptide motif which is part of the active site strand-turn-helix motif. In SbHNL, however, a unique two-amino acid deletion is next to the putative active site Ser158, removing thereby the putative oxyanion hole-forming Tyr residue. The presented X-ray structure shows that the overall folding pattern of SbHNL is similar to that of the closely related wheat serine carboxypeptidase (CPD-WII); however, the deletion in SbHNL is forcing the putative active site residues away from the expected hydrolase binding site toward a small hydrophobic cleft, which also contains the inhibitor benzoic acid, defining thereby a completely different SbHNL active site architecture where the traditional view of a classic triad is not given any more. Rather, we propose a mechanism involving general base catalysis by the carboxy-terminal Trp270 carboxyl group and proton transfer toward the leaving nitrile group by an active site water molecule. The unexpected interactions of the inhibitor with the new SbHNL active site also reveal the structural basis for the enzyme's limited substrate specificity. The implications of this structure on the evolution of catalysis in the hydroxynitrile lyase superfamily are discussed.
Organizational Affiliation:
Institut für Organische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D-70569 Stuttgart, Germany. PeterLauble@t-online.de