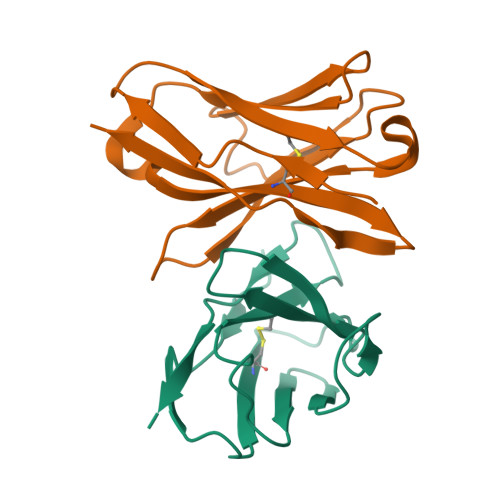
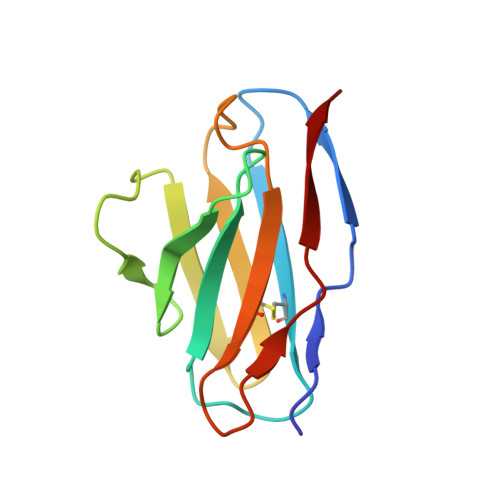
Structure of an anti-blood group A Fv and improvement of its binding affinity without loss of specificity.
Thomas, R., Patenaude, S.I., MacKenzie, C.R., To, R., Hirama, T., Young, N.M., Evans, S.V.(2002) J Biological Chem 277: 2059-2064
- PubMed: 11679577
- DOI: https://doi.org/10.1074/jbc.M104364200
- Primary Citation of Related Structures:
1JV5 - PubMed Abstract:
The specificity of antibody recognition of the ABO blood group trisaccharide antigens has been explored by crystal structure analysis and mutation methods. The crystal structure of the Fv corresponding to the anti-blood group A antibody AC1001 has been determined to 2.2-A resolution and reveals a binding pocket that is complementary to the blood group A-trisaccharide antigen. The effect of mutating specific residues lining this pocket on binding to the A and B blood group oligosaccharide antigens was investigated through a panel of single point mutations and through a phage library of mutations in complementarity determining region H3. Both approaches gave several mutants with improved affinity for antigen. Surface plasmon resonance indicated up to 8-fold enhancement in affinity for the A-pentasaccharide with no observable binding to the blood group B antigen. This is the first example of single point mutations in a carbohydrate-binding antibody resulting in significant increases in binding affinity without loss of specificity.
Organizational Affiliation:
Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, Ontario K1H 8M5, Canada.