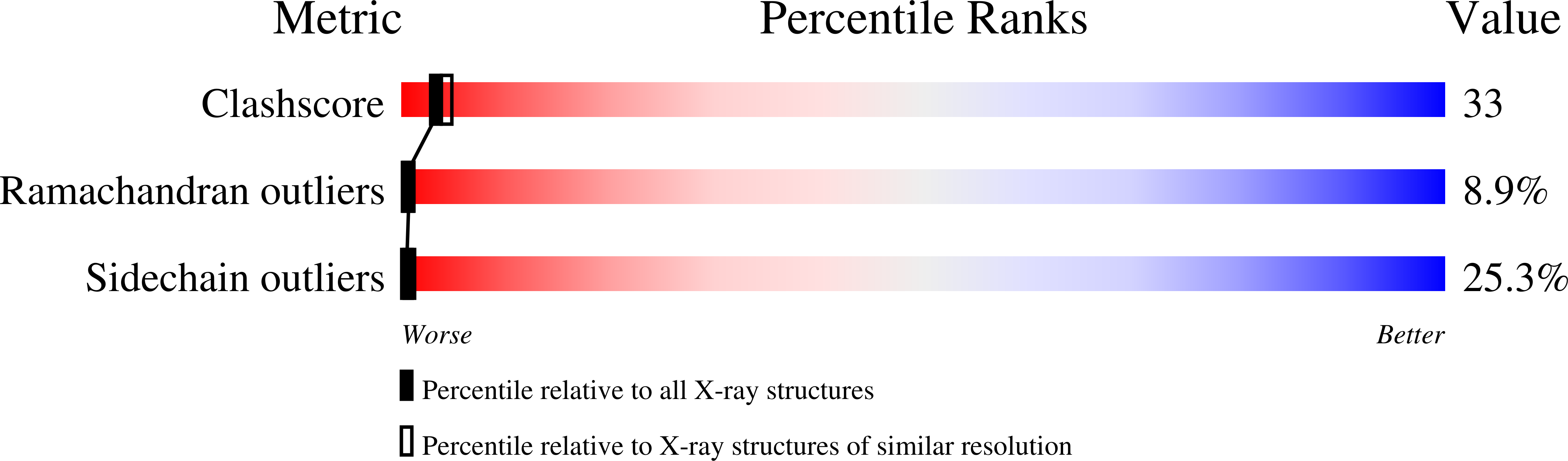Structure of bovine adenosine deaminase complexed with 6-hydroxy-1,6-dihydropurine riboside.
Kinoshita, T., Nishio, N., Nakanishi, I., Sato, A., Fujii, T.(2003) Acta Crystallogr D Biol Crystallogr 59: 299-303
- PubMed: 12554940
- DOI: https://doi.org/10.1107/s090744490202190x
- Primary Citation of Related Structures:
1KRM - PubMed Abstract:
The crystal structure of adenosine deaminase (ADA) from bovine intestine complexed with a transition-state analogue, 6-hydroxy-1,6-dihydropurine riboside (HDPR), was solved at 2.5 A resolution by the molecular-replacement method using a homology model based on the crystal structure of mouse ADA. The final refinement converged to a crystallographic R factor of 20.7%. The C(alpha) backbone of bovine ADA is mostly superimposable on that of mouse ADA, although mouse ADA itself did not lead to a solution by molecular replacement. HDPR tightly interacts with ADA by means of six hydrogen bonds and is entirely enclosed within the active site. The lid of the envelope consists of two components: one contains two leucine residues, Leu55 and Leu59, and the other contains the backbone atoms Asp182 and Glu183. The C(delta) atoms of the two leucine residues are 3.5 A from the respective N atoms of the backbone. A weak interaction, similar to CH-pi binding, might make it possible to open the lid. Taking account of the movement and observation of this structural feature, the aim is to design novel ADA inhibitors.
Organizational Affiliation:
Exploratory Research Laboratories, Fujisawa Pharmaceutical Co. Ltd, 5-2-3 Tokodai, Tsukuba, Ibaraki 300-2698, Japan. takayoshi_kinoshita@po.fujisawa.co.jp