Steroid Ligands Bind Human Sex Hormone-binding Globulin in Specific Orientations and Produce Distinct Changes in Protein Conformation
Grishkovskaya, I., Avvakumov, G.V., Hammond, G.L., Catalano, M.G., Muller, Y.A.(2002) J Biological Chem 277: 32086-32093
- PubMed: 12065592
- DOI: https://doi.org/10.1074/jbc.M203999200
- Primary Citation of Related Structures:
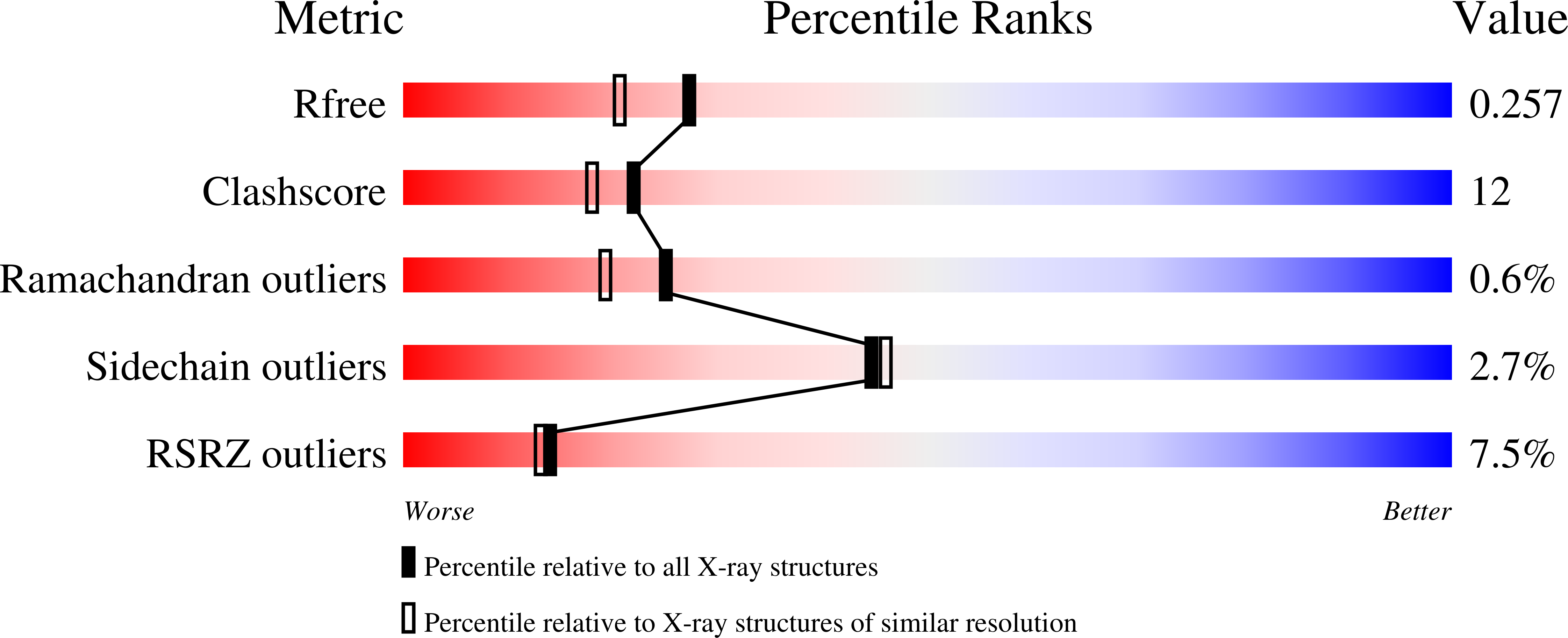
1LHN, 1LHO, 1LHU, 1LHV - PubMed Abstract:
The amino-terminal laminin G-like domain of human sex hormone-binding globulin (SHBG) contains a single high affinity steroid-binding site. Crystal structures of this domain in complex with several different steroid ligands have revealed that estradiol occupies the SHBG steroid-binding site in an opposite orientation when compared with 5 alpha-dihydrotestosterone or C19 androgen metabolites (5 alpha-androstan-3 beta,17 beta-diol and 5 alpha-androstan-3 beta,17 alpha-diol) or the synthetic progestin levonorgestrel. Substitution of specific residues within the SHBG steroid-binding site confirmed that Ser(42) plays a key role in determining high affinity interactions by hydrogen bonding to functional groups at C3 of the androstanediols and levonorgestrel and the hydroxyl at C17 of estradiol. Among residues participating in the hydrogen bond network with hydroxy groups at C17 of C19 steroids or C3 of estradiol, Asp(65) appears to be the most important. The different binding mode of estradiol is associated with a difference in the position/orientation of residues (Leu(131) and Lys(134)) in the loop segment (Leu(131)-His(136)) that covers the steroid-binding site as well as others (Leu(171)-Lys(173) and Trp(84)) on the surface of human SHBG and may provide a basis for ligand-dependent interactions between SHBG and other macromolecules. These new crystal structures have also enabled us to construct a simple space-filling model that can be used to predict the characteristics of novel SHBG ligands.
Organizational Affiliation:
Forschungsgruppe Kristallographie, Max-Delbrück-Centrum für Molekulare Medizin, D-13092 Berlin, Germany.