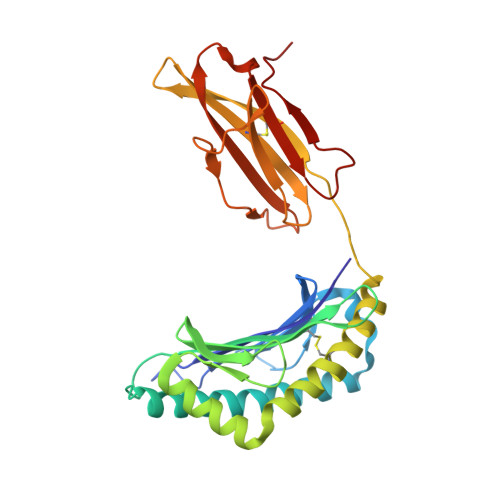
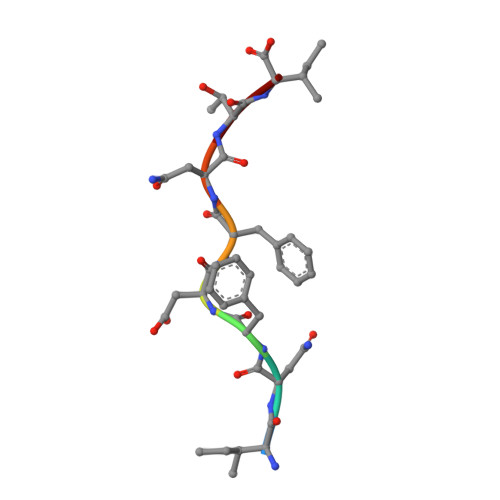
CDR3 loop flexibility contributes to the degeneracy of TCR recognition
Reiser, J.-B., Darnault, C., Gregoire, C., Mosser, T., Mazza, G., Kearnay, A., van der Merwe, P.A., Fontecilla-Camps, J.C., Housset, D., Malissen, B.(2003) Nat Immunol 4: 241-247
- PubMed: 12563259
- DOI: https://doi.org/10.1038/ni891
- Primary Citation of Related Structures:
1NAM, 1NAN - PubMed Abstract:
T cell receptor (TCR) binding degeneracy lies at the heart of several physiological and pathological phenomena, yet its structural basis is poorly understood. We determined the crystal structure of a complex involving the BM3.3 TCR and an octapeptide (VSV8) bound to the H-2K(b) major histocompatibility complex molecule at a 2.7 A resolution, and compared it with the BM3.3 TCR bound to the H-2K(b) molecule loaded with a peptide that has no primary sequence identity with VSV8. Comparison of these structures showed that the BM3.3 TCR complementarity-determining region (CDR) 3alpha could undergo rearrangements to adapt to structurally different peptide residues. Therefore, CDR3 loop flexibility helps explain TCR binding cross-reactivity.
Organizational Affiliation:
Laboratoire de Cristallographie et Cristallogenèse des Protéines, Institut de Biologie Structurale J.-P. Ebel, CEA-CNRS-UJF, 41 rue Jules Horowitz, F-38027 Grenoble Cedex 1, France.