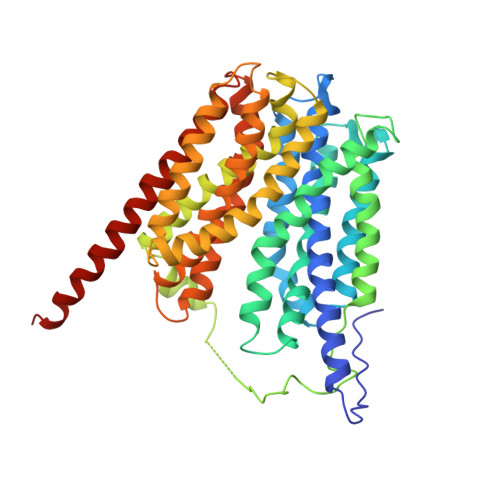
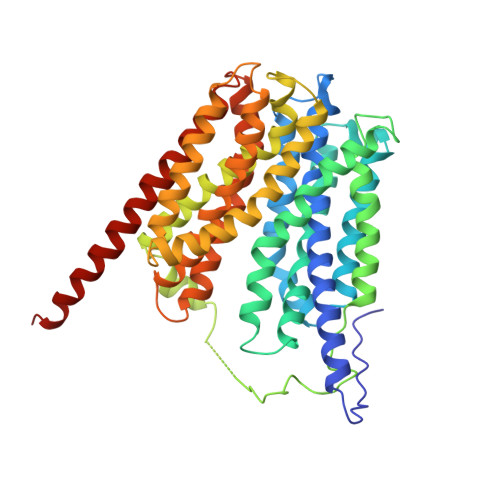
Structure and Mechanism of the Glycerol-3-Phosphate Transporter from Escherichia Coli
Huang, Y., Lemieux, M.J., Song, J., Auer, M., Wang, D.N.(2003) Science 301: 616-620
- PubMed: 12893936
- DOI: https://doi.org/10.1126/science.1087619
- Primary Citation of Related Structures:
1PW4 - PubMed Abstract:
The major facilitator superfamily represents the largest group of secondary membrane transporters in the cell. Here we report the 3.3 angstrom resolution structure of a member of this superfamily, GlpT, which transports glycerol-3-phosphate into the cytoplasm and inorganic phosphate into the periplasm. The amino- and carboxyl-terminal halves of the protein exhibit a pseudo two-fold symmetry. Closed off to the periplasm, a centrally located substrate-translocation pore contains two arginines at its closed end, which comprise the substrate-binding site. Upon substrate binding, the protein adopts a more compact conformation. We propose that GlpT operates by a single-binding site, alternating-access mechanism through a rocker-switch type of movement.
Organizational Affiliation:
Skirball Institute of Biomolecular Medicine and Department of Cell Biology, New York University School of Medicine, 540 First Avenue, New York, NY 10016, USA.