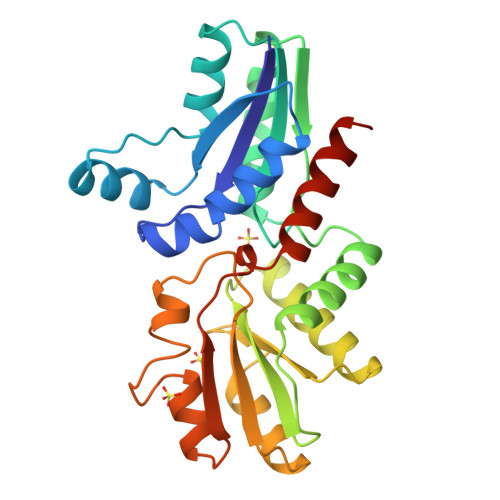
Common chelatase design in the branched tetrapyrrole pathways of heme and anaerobic cobalamin synthesis.
Schubert, H.L., Raux, E., Wilson, K.S., Warren, M.J.(1999) Biochemistry 38: 10660-10669
- PubMed: 10451360
- DOI: https://doi.org/10.1021/bi9906773
- Primary Citation of Related Structures:
1QGO - PubMed Abstract:
Prosthetic groups such as heme, chlorophyll, and cobalamin (vitamin B(12)) are characterized by their branched biosynthetic pathway and unique metal insertion steps. The metal ion chelatases can be broadly classed either as single-subunit ATP-independent enzymes, such as the anaerobic cobalt chelatase and the protoporphyrin IX (PPIX) ferrochelatase, or as heterotrimeric, ATP-dependent enzymes, such as the Mg chelatase involved in chlorophyll biosynthesis. The X-ray structure of the anaerobic cobalt chelatase from Salmonella typhimurium, CbiK, has been solved to 2.4 A resolution. Despite a lack of significant amino acid sequence similarity, the protein structure is homologous to that of Bacillus subtilis PPIX ferrochelatase. Both enzymes contain a histidine residue previously identified as the metal ion ligand, but CbiK contains a second histidine in place of the glutamic acid residue identified as a general base in PPIX ferrochelatase. Site-directed mutagenesis has confirmed a role for this histidine and a nearby glutamic acid in cobalt binding, modulating metal ion specificity as well as catalytic efficiency. Contrary to the predicted protoporphyrin binding site in PPIX ferrochelatase, the precorrin-2 binding site in CbiK is clearly defined within a large horizontal cleft between the N- and C-terminal domains. The structural similarity has implications for the understanding of the evolution of this branched biosynthetic pathway.
Organizational Affiliation:
Department of Chemistry, University of York, U.K.