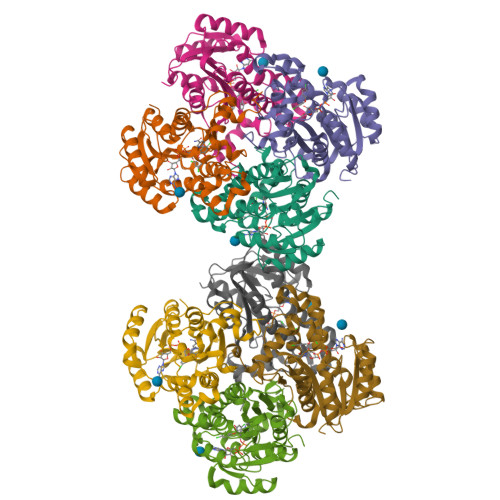
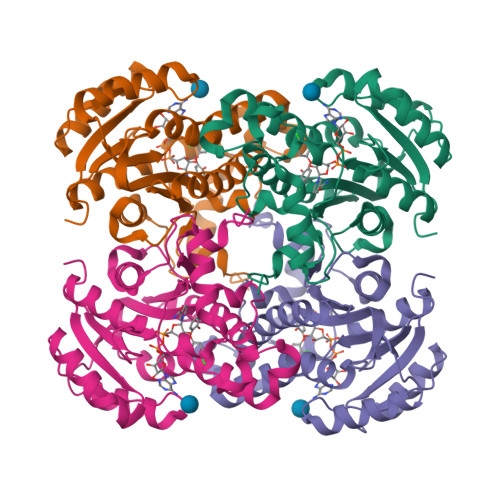
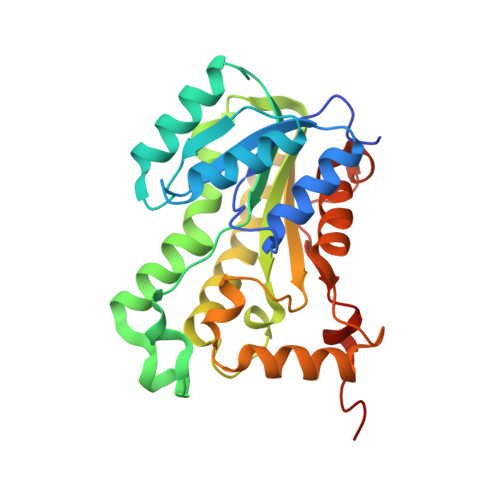
Structural basis and mechanism of enoyl reductase inhibition by triclosan.
Stewart, M.J., Parikh, S., Xiao, G., Tonge, P.J., Kisker, C.(1999) J Mol Biology 290: 859-865
- PubMed: 10398587
- DOI: https://doi.org/10.1006/jmbi.1999.2907
- Primary Citation of Related Structures:
1QSG - PubMed Abstract:
The enoyl-acyl carrier protein reductase (ENR) is involved in bacterial fatty acid biosynthesis and is the target of the antibacterial diazaborine compounds and the front-line antituberculosis drug isoniazid. Recent studies suggest that ENR is also the target for the broad-spectrum biocide triclosan. The 1.75 A crystal structure of EnvM, the ENR from Escherichia coli, in complex with triclosan and NADH reveals that triclosan binds specifically to EnvM. These data provide a molecular mechanism for the antibacterial activity of triclosan and substantiate the hypothesis that its activity results from inhibition of a specific cellular target rather than non-specific disruption of the bacterial cell membrane. This has important implications for the emergence of drug-resistant bacteria, since triclosan is an additive in many personal care products such as toothpastes, mouthwashes and soaps. Based on this structure, rational design of triclosan derivatives is possible which might be effective against recently identified triclosan-resistant bacterial strains.
Organizational Affiliation:
Department of Pharmacological Sciences, SUNY at Stony Brook, Stony Brook, NY, 11794-8651, USA.