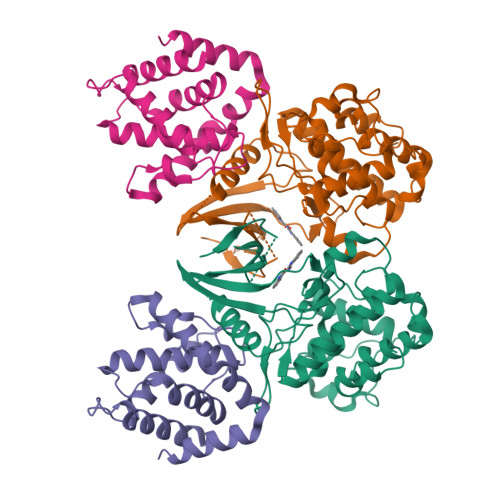
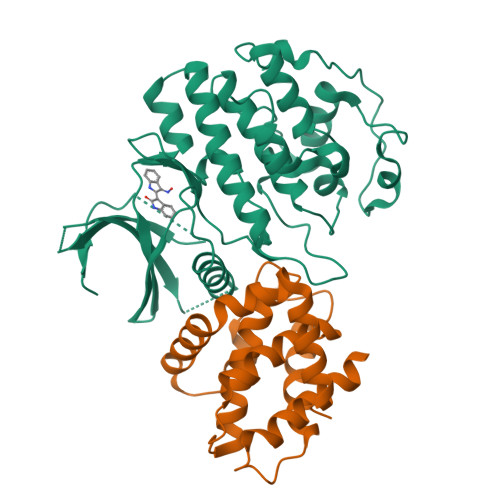
Mechanism of Cdk5/P25 Binding by Cdk Inhibitors
Mapelli, M., Massimilinao, L., Crovace, C., Seeliger, M.A., Tsai, L.-H., Meijer, L., Musacchio, A.(2005) J Med Chem 48: 671
- PubMed: 15689152
- DOI: https://doi.org/10.1021/jm049323m
- Primary Citation of Related Structures:
1UNG, 1UNH, 1UNL - PubMed Abstract:
The cyclin-dependent kinases (CDK) CDK1, CDK2, CDK4, and CDK6 are serine/threonine protein kinases targeted in cancer therapy due to their role in cell cycle progression. The postmitotic CDK5 is involved in biological pathways important for neuronal migration and differentiation. CDK5 represents an attractive pharmacological target as its deregulation is implicated in various neurodegenerative diseases such as Alzheimer's, Parkinson's, and Niemann-Pick type C diseases, ischemia, and amyotrophic lateral sclerosis. We have generated an improved crystal form of CDK5 in complex with p25, a segment of the p35 neuronal activator. The crystals were used to solve the structure of CDK5/p25 with (R)-roscovitine and aloisine at a resolution of 2.2 and 2.3 A, respectively. The structure of CDK5/p25/roscovitine provides a rationale for the preference of CDK5 for the R over the S stereoisomer. Furthermore, roscovitine stabilized an unusual collapsed conformation of the glycine-rich loop, an important site of CDK regulation, and we report an investigation of the effects of glycine-rich loop phosphorylation on roscovitine binding. The CDK5/p25 crystals represent a valuable new tool for the identification and optimization of selective CDK inhibitors.
Organizational Affiliation:
Structural Biology Unit, Department of Experimental Oncology, European Institute of Oncology, Via Ripamonti 435, 20141 Milan, Italy.