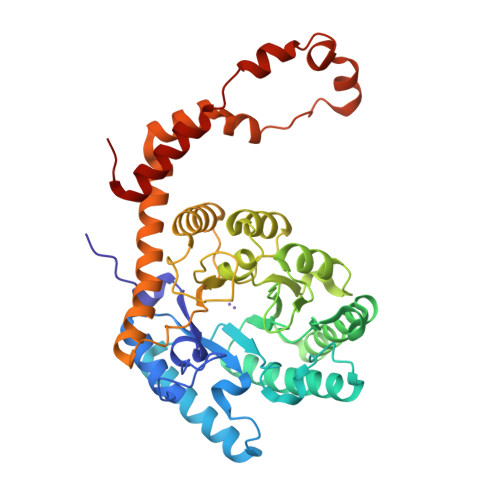
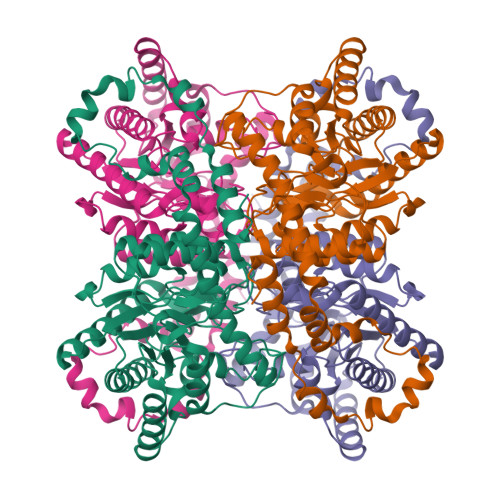
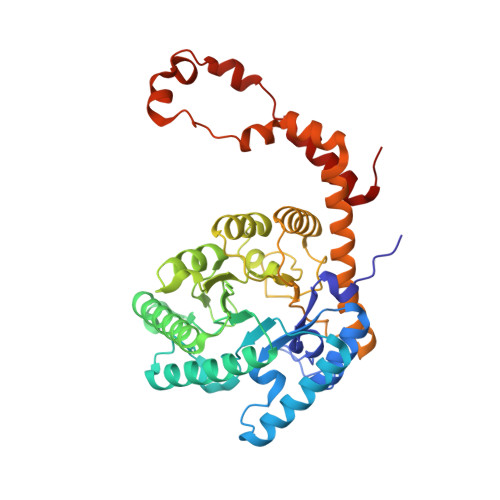
Modes of binding substrates and their analogues to the enzyme D-xylose isomerase.
Carrell, H.L., Hoier, H., Glusker, J.P.(1994) Acta Crystallogr D Biol Crystallogr 50: 113-123
- PubMed: 15299449
- DOI: https://doi.org/10.1107/S0907444993009345
- Primary Citation of Related Structures:
1XIB, 1XIC, 1XID, 1XIE, 1XIF, 1XIG, 1XIH, 1XII, 1XIJ - PubMed Abstract:
Studies of binding of substrates and inhibitors of the enzyme D-xylose isomerase show, from X-ray diffraction data at 1.6-1.9 A resolution, that there are a variety of binding modes. These vary in the manner in which the substrate or its analogue extend, on binding, across the carboxy end of the (betaalpha)(8)-barrel structure. These binding sites are His54 and the metal ion (magnesium or manganese) that is held in place by Glul81, Asp245, Glu217 and Asp287. Possible catalytic groups have been identified in proposed mechanisms and their role in the binding of ligands is illustrated.
Organizational Affiliation:
The Institute for Cancer Research, The Fox Chase Cancer Center, Philadelphia, PA 19111, USA.