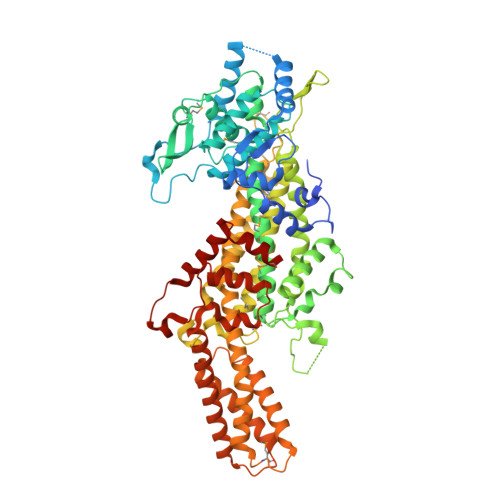
Structure-based chemical modification strategy for enzyme replacement treatment of phenylketonuria.
Wang, L., Gamez, A., Sarkissian, C.N., Straub, M., Patch, M.G., Han, G.W., Striepeke, S., Fitzpatrick, P., Scriver, C.R., Stevens, R.C.(2005) Mol Genet Metab 86: 134-140
- PubMed: 16006165
- DOI: https://doi.org/10.1016/j.ymgme.2005.05.012
- Primary Citation of Related Structures:
1Y2M - PubMed Abstract:
Structure-based protein engineering coupled with chemical modifications (e.g., pegylation) is a powerful combination to significantly improve the development of proteins as therapeutic agents. As a test case, phenylalanine ammonia-lyase (PAL, EC 4.3.1.5) was selected for enzyme replacement therapy in phenylketonuria [C.R. Scriver, S. Kaufman, Hyperphenylalaninemia:phenylalanine Hydroxylase Deficiency. The Metabolic and Molecular Bases of Inherited Disease, McGraw-Hill, New York, 2001, Chapter 77], an inherited metabolic disorder (OMIM 261600) causing mental retardation due to deficiency of the enzyme l-phenylalanine hydroxylase (EC 1.14.16.1). Previous in vivo studies of recombinant PAL demonstrated a lowering of blood l-phenylalanine levels; yet, the metabolic effect was not sustained due to protein degradation and immunogenicity [C.N. Sarkissian, Z. Shao, F. Blain, R. Peevers, H. Su, R. Heft, T.M. Chang, C.R. Scriver, A different approach to treatment of phenylketonuria:phenylalanine degradation with recombinant phenylalanine ammonia lyase, Proc. Natl. Acad. Sci. USA 96 (1999) 2339; J.A. Hoskins, G. Jack, H.E. Wade, R.J. Peiris, E.C. Wright, D.J. Starr, J. Stern, Enzymatic control of phenylalanine intake in phenylketonuria, Lancet 1 (1980) 392; C.M. Ambrus, S. Anthone, C. Horvath, K. Kalghatgi, A.S. Lele, G. Eapen, J.L. Ambrus, A.J. Ryan, P. Li, Extracorporeal enzyme reactors for depletion of phenylalanine in phenylketonuria, Ann. Intern. Med. 106 (1987) 531]. Here, we report the 1.6A three-dimensional structure of Rhodosporidium toruloides PAL, structure-based molecular engineering, pegylation of PAL, as well as in vitro and in vivo PKU mouse model studies on pegylated PAL formulations. Our results show that pegylation of R. toruloides PAL leads to promising therapeutic efficacy after subcutaneous injection by enhancing the in vivo activity, lowering plasma phenylalanine, and leading to reduced immunogenicity. The three-dimensional structure of PAL provides a basis for understanding the properties of pegylated forms of PAL and strategies for structure-based re-engineering of PAL for PKU treatment.
Organizational Affiliation:
Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.